Cho 4g copper(II) oxide(CuO) tác dụng với khí hydrogen. Tạo ra 2g copper. Tính hiệu suất phản ứng
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\)
PTHH:
Mg + 2HCl ---> MgCl2 + H2
0,3-->0,6----------------->0,3
=> \(\left\{{}\begin{matrix}V_{H_2}=24,79.0,3=7,437\left(l\right)\\m_{HCl}=0,6.36,5=21,9\left(g\right)\end{matrix}\right.\)
\(n_{CuO}=\dfrac{12}{80}=0,15\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
LTL: 0,15 < 0,3 => H2 dư, vậy H2 khử hết CuO
a, \(n_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\)
Mg + 2HCl -----> MgCl2 + H2
0,3 0,6 0,3
\(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
b, \(m_{HCl}=0,6.36,5=21,9\left(g\right)\)
c, \(n_{CuO}=\dfrac{12}{80}=0,15\left(mol\right)\)
CuO + H2 -----> Cu + H2O
Ta có: \(\dfrac{0,15}{1}< \dfrac{0,3}{1}\) ⇒ CuO hết, H2 dư

a, \(2Cu\left(NO_3\right)_2\underrightarrow{t^o}2CuO+4NO_2+O_2\)
b, \(n_{Cu\left(NO_3\right)_2}=\dfrac{28,2}{188}=0,15\left(mol\right)\)
Theo PT: \(\left\{{}\begin{matrix}n_{CuO}=n_{Cu\left(NO_3\right)_2}=0,15\left(mol\right)\\n_{O_2}=\dfrac{1}{2}n_{Cu\left(NO_3\right)_2}=0,075\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{CuO}=0,15.80=12\left(g\right)\)
\(V_{O_2}=0,075.24,79=1,85925\left(l\right)\)
c, Ta có: \(n_{NO_2}+n_{O_2}=\dfrac{6,1975}{24,79}=0,25\left(mol\right)\)
Gọi: nO2 = x (mol)
Theo PT: \(n_{NO_2}=4n_{O_2}=4x\left(mol\right)\)
⇒ 4x + x = 0,25 ⇒ x = 0,05 (mol)
Theo PT: \(n_{Cu\left(NO_3\right)_2\left(LT\right)}=2n_{O_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{Cu\left(NO_3\right)_2\left(LT\right)}=0,1.188=18,8\left(g\right)\)
Mà: H = 80% \(\Rightarrow m_{Cu\left(NO_3\right)_2\left(TT\right)}=\dfrac{18,8}{80\%}=23,5\left(g\right)\)

nFe = 11,2/56 = 0,2 (mol)
PTHH: Fe + 2HCl -> FeCl2 + H2
Mol: 0,2 ---> 0,4 ---> 0,2 ---> 0,2
VH2 = 0,2 . 22,4 = 4,48 (l)
PTHH: CuO + H2 -> (to) Cu + H2O
Mol: 0,2 <--- 0,2 ---> 0,2
mCu = 0,2 . 64 = 12,8 (g)

a, PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Ta có: \(n_{CuO}=\dfrac{12}{80}=0,15\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Cu}=n_{H_2O}=n_{CuO}=0,15\left(mol\right)\)
b, \(m_{Cu}=0,15.64=9,6\left(g\right)\)
\(m_{H_2O}=0,15.18=2,7\left(g\right)\)
c, \(V_{H_2}=0,15.24,79=3,7185\left(l\right)\)

Bài 9 :
\(n_{CuO}=\dfrac{4}{80}=0,05\left(mol\right)\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
0,05--->0,1-------->0,05
a) \(C_{MddHCl}=\dfrac{0,1}{0,1}=1\left(M\right)\)
b) \(m_{CuCl2}=0,05.135=6,75\left(g\right)\)
c) \(C_{MCuCl2}=\dfrac{0,05}{0,1}0,5\left(M\right)\)
Câu 10 :
\(n_{FeO}=\dfrac{3,6}{72}=0,05\left(mol\right)\)
\(FeO+2HCl\rightarrow FeCl_2+H_2O\)
0,05-->0,1------->0,05
\(m_{ddHCl}=\dfrac{0,1.36,5}{10\%}100\%=36,5\left(g\right)\)
\(m_{ddspu}=3,6+36,5=40,1\left(g\right)\)
\(C\%_{FeCl2}=\dfrac{0,05.127}{40,1}.100\%=15,84\%\)

\(2KClO_3\xrightarrow[xtMnO_2]{t^o}2KCl+3O_2\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\\
H_2+CuO\underrightarrow{400^oC}H_2O+Cu\\
CaO+H_2O\rightarrow Ca\left(OH\right)_2\)
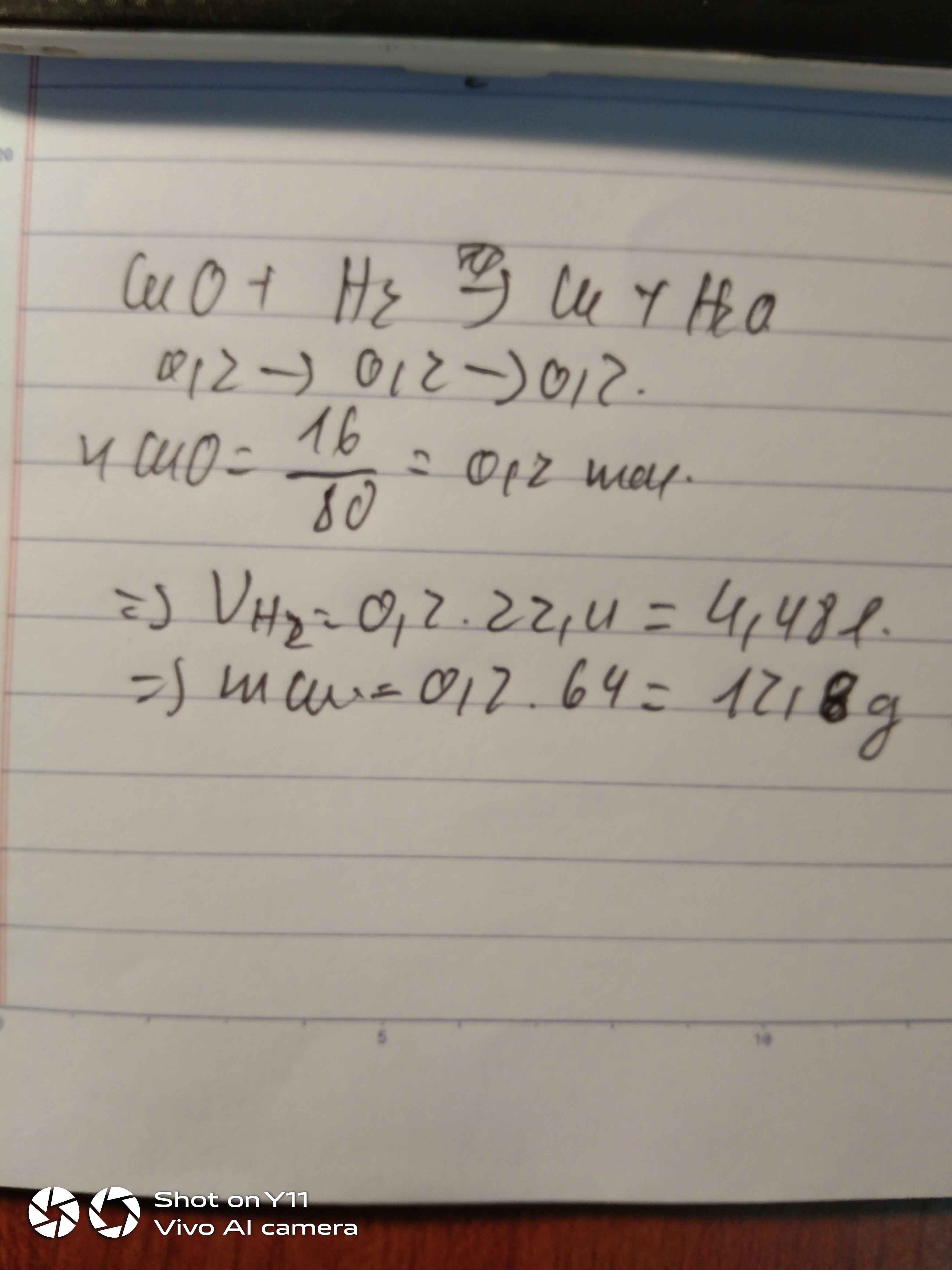
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\\ n_{Cu\left(LT\right)}=n_{CuO}=\dfrac{4}{80}=0,05\left(mol\right)\\ n_{Cu\left(TT\right)}=\dfrac{2}{64}=0,03125\left(mol\right)\\ \Rightarrow H=\dfrac{0,03125}{0,05}.100\%=62,5\%\)