Tính phân tử khối của Mg(OH)2, Ca(H2PO4)2, Ba3(PO4)2, Al2(SO4)3, Ca(HCO3)2, Fe(NO3)2
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


“Phân tử khối bằng tổng khối lượng của các nguyên tửu trong phân tử”
A l 2 O 3 (M = 27.2 + 16.3 = 102 đvC )
A l 2 ( S O 4 ) 3 (M = 342 đvC ) F e ( N O 3 ) 3 ( M = 242 đvC )
N a 3 P O 4 (M = 164 đvC ) C a ( H 2 P O 4 ) 2 ( M = 234 đvC )
B a 3 ( P O 4 ) 2 (M = 601 đvC ) Z n S O 4 ( M = 161 đvC )
AgCl (M = 143,5 đvC ) NaBr ( M = 103 đvC )

1. \(2Al+3Fe\left(NO_3\right)_2\rightarrow2Al\left(NO_3\right)_3+3Fe\)
2. \(P_2O_5+3Ba\left(OH\right)_2\rightarrow Ba_3\left(PO_4\right)_2+3H_2O\)
3. \(Al\left(OH\right)_3+3HCl\rightarrow AlCl_3+3H_2O\)
4. \(Al_2\left(SO_4\right)_3+3Ca\left(OH\right)_2\rightarrow3CaSO_4+2Al\left(OH\right)_3\)
5. \(Fe_3O_4+8HCl\rightarrow FeCl_2+2FeCl_3+4H_2O\)
6. \(Mg\left(NO_3\right)_2\underrightarrow{t^o}MgO+2NO_2+\dfrac{1}{2}O_2\)
7. \(2xFe+yO_2\underrightarrow{t^o}2Fe_xO_y\)
8. \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
9. \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
Bạn tham khảo nhé!
\(1.Al+Fe\left(NO_3\right)_2--->Al\left(NO_3\right)_2+Fe\)
\(2.P_2O_5+3Ba\left(OH\right)_2--->Ba_3\left(PO_4\right)_2+3H_2O\)
\(3.Al\left(OH\right)_3+3HCl--->AlCl_3+3H_2O\)
\(4.Al_2\left(SO_4\right)_3+3Ca\left(OH\right)_2--->3CaSO_4+2Al\left(OH\right)_3\downarrow\)
\(5.Fe_3O_4+8HCl--->FeCl_2+2FeCl_3+4H_2O\)
\(6.2Mg\left(NO_3\right)_2\overset{t^o}{--->}2MgO+4NO_2+O_2\)
\(7.xFe+\dfrac{y}{2}O_2\overset{t^o}{--->}Fe_xO_y\)
\(8.CH_4+2O_2\overset{t^o}{--->}CO_2+2H_2O\)
\(9.C_2H_6O+3O_2\overset{t^o}{--->}2CO_2+3H_2O\)

\(1,\left\{{}\begin{matrix}p=e\\n+p+e=40\\2p-n=12\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}2p+n=40\\2p-n=12\end{matrix}\right.\Leftrightarrow n=\dfrac{40-12}{2}=14\)
\(2,PTK_{Al_2O_3}=2\cdot27+16\cdot3=102\left(đvC\right)\\ PTK_{Al_2\left(SO_4\right)_3}=2\cdot27+\left(32+16\cdot4\right)\cdot3=342\left(đvC\right)\\ PTK_{Fe\left(NO_3\right)_3}=56+\left(14+16\cdot3\right)\cdot3=242\left(đvV\right)\\ PTK_{Na_3PO_4}=23\cdot3+31+16\cdot4=164\left(đvC\right)\\ PTK_{Ca\left(H_2PO_4\right)_2}=40+\left(2+31+16\cdot4\right)\cdot2=234\left(đvC\right)\\ PTK_{Ba_3PO_4}=137\cdot3+31+16\cdot4=506\left(đvC\right)\\ PTK_{ZnSO_4}=65+32+16\cdot4=161\left(đvC\right)\\ PTK_{AgCl}=108+35,5=143,5\left(đvC\right)\\ PTK_{NaBr}=23+80=103\left(đvC\right)\)

Câu 1:
O: 6 e ngoài cùng
N: 4 e ngoài cùng
K:1 e ngoài cùng
P: 5 e ngoài cùng
Câu 2:
\(PTK_{Al_2O_3}=2.27+16.3=102\left(đVc\right)\)
Al2(SO4)3=27.2+3.(32+16.4)=342(đVc)
\(Fe\left(NO_3\right)_3=56+3.\left(14+16.3\right)=242\left(đVc\right)\)
\(Na_3PO_4=23.3+31+16.4=164\left(đVc\right)\)
\(Ca\left(H_2PO_4\right)_2=40+2.\left(1.2+31+16.4\right)=234\left(đVc\right)\)
\(Ba_3\left(PO_4\right)_2=137.3+2.\left(31+16.4\right)=601\left(đVc\right)\)
\(ZnSO_4=65+32+16.4=161\left(đVc\right)\)
\(AgCl=108+35,5=143,5\left(đVc\right)\)
\(NaBr=23+80=103\left(đVc\right)\)

$a) CaO + H_2O \to Ca(OH)_2$
$b) NaOH + HCl \to NaCl + H_2O$
$c) Ca(OH)_2 + 2HNO_3 \to Ca(NO_3)_2 + 2H_2O$
$d) AlCl_3 + 3NaOH \to Al(OH)_3 + 3NaCl$
$e) Mg(NO_3)_2 + Ca(OH)_2 \to Ca(NO_3)_2 + Mg(OH)_2$
$f) CuCl_2 + 2KOH \to Cu(OH)_2 + 2KCl$
$g) Fe(OH)_2 + H_2SO_4 \to FeSO_4 + 2H_2O$
$h) 2Fe(OH)_3 + 3H_2SO_4 \to Fe_2(SO_4)_3 + 6H_2O$
$i) Al_2(SO_4)_3 + 3BaCl_2 \to 3BaSO_4 + 2AlCl_3$

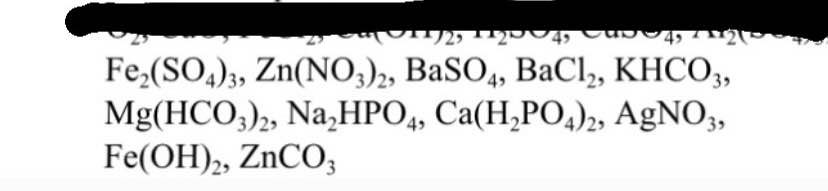
\(PTK_{Mg\left(OH\right)_2}=24+\left(16+1\right).2=58\left(đvC\right)\)
\(PTK_{Ca\left(H_2PO_4\right)_2}=40+\left(1.2+31+16.4\right).2=234\left(đvC\right)\)
\(PTK_{Ba_3\left(PO_4\right)_2}=137.3+\left(31+16.4\right).2=601\left(đvC\right)\)
\(PTK_{Al_2\left(SO_4\right)_3}=27.2+\left(32+16.4\right).3=342\left(đvC\right)\)
\(PTK_{Ca\left(HCO_3\right)_2}=40+\left(1+12+16.3\right).2=162\left(đvC\right)\)
\(PTK_{Fe\left(NO_3\right)_2}=56+\left(14+16.3\right).2=180\left(đvC\right)\)
\(PTK_{Mg\left(OH\right)_2}=24+\left(16+1\right)\cdot2=58\left(đvC\right)\\ PTK_{Ca\left(H_2PO_4\right)_2}=40+\left(2+31+16\cdot4\right)\cdot2=234\left(đvC\right)\\ PTK_{Ba_3\left(PO_4\right)_2}=137\cdot3+\left(31+16\cdot4\right)\cdot2=601\left(đvC\right)\\ PTK_{Al_2\left(SO_4\right)_3}=27\cdot2+\left(32+16\cdot4\right)\cdot3=342\left(đvC\right)\\ PTK_{Ca\left(HCO_3\right)_2}=40+\left(1+12+16\cdot3\right)\cdot2=162\left(đvC\right)\\ PTK_{Fe\left(NO_3\right)_2}=56+\left(14+16\cdot3\right)\cdot2=180\left(đvC\right)\)