Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1:
PTHH: \(C_2H_5OH+O_2\xrightarrow[]{mengiấm}CH_3COOH+H_2O\)
Ta có: \(n_{C_2H_5OH}=\dfrac{115\cdot0,8}{46}=2\left(mol\right)=n_{CH_3COOH\left(lýthuyết\right)}\)
\(\Rightarrow m_{CH_3COOH\left(thực\right)}=2\cdot60\cdot90\%=108\left(g\right)\)
Bài 2:
PTHH: \(C_2H_5OH+CH_3COOH\xrightarrow[H_2SO_4\left(đ\right)]{t^o}CH_3COOC_2H_5+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CH_3COOH}=\dfrac{60}{60}=1\left(mol\right)\\n_{C_2H_5OH}=\dfrac{92}{46}=2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Rượu còn dư, Axit p/ứ hết
\(\Rightarrow n_{CH_3COOC_2H_5\left(lýthuyết\right)}=1\left(mol\right)\) \(\Rightarrow m_{CH_3COOC_2H_5\left(thực\right)}=1\cdot88\cdot80\%=70,4\left(g\right)\)

a, \(n_{C_2H_4}=\dfrac{15,68}{22,4}=0,7\left(mol\right)\)
PT: \(C_2H_4+H_2O\underrightarrow{^{t^o,xt}}C_2H_5OH\)
Theo PT: \(n_{C_2H_5OH\left(LT\right)}=n_{C_2H_4}=0,7\left(mol\right)\)
Mà: H = 90%
\(\Rightarrow n_{C_2H_5OH\left(TT\right)}=0,7.90\%=0,63\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH\left(TT\right)}=0,63.46=28,98\left(g\right)\)
\(\Rightarrow V_{C_2H_5OH}=\dfrac{28,98}{0,8}=36,225\left(ml\right)\)
b, \(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
Theo PT: \(n_{CH_3COOH}=n_{C_2H_5OH}=0,63\left(mol\right)\)
\(\Rightarrow m_{CH_3COOH}=0,63.60=37,8\left(g\right)\)
\(\Rightarrow m_{ddCH_3COOH}=\dfrac{37,8}{5\%}=756\left(g\right)\)

a)
$C_2H_5OH + CH_3COOH \buildrel{{H_2SO_4,t^o}}\over\rightleftharpoons CH_3COOC_2H_5 + H_2O$
b)
n CH3COOC2H5 = n C2H5OH = 9,2/46 = 0,2(mol)
=> m este = 0,2.88 = 17,6 gam
c)
n este = 8,8/88 = 0,1(mol)
=> n C2H5OH = n CH3COOH = 0,1/60% = 1/6 mol
=> m C2H5OH = 46 . 1/6 = 7,67(gam) ; m CH3COOH = 60 . 1/6 = 10(gam)

a, Vdd= 1,25 lít = 125 ml
Thể tích rượu etylic nguyên chất đã dùng là :
V\(_r\) = \(\frac{Vdd\cdotĐr}{100}\)= \(\frac{125\cdot20}{100}\) = 25 (ml)
b, m\(_r\)= D* V\(_r\) = 0,8 * 25 = 20 gam
hay mC2H5OH = m\(_r\) = 20 gam
nC2H5OH = 20/ 46 = 10/23 (mol)
C2H5OH + O2 → CH3COOH + H2O ( men giấm )
(mol) 10/23 → 10/23
mCH3COOH = 10/23 * 60 ~ 26,1(gam)
c, nAl = 270/ 27 = 10(mol)
2Al + 6CH3COOH → 2(CH3COO)3Al + 3H2O
(mol) 10 10/23
xét tỉ lệ : 10/2 > (10/23)/6
PƯ : 10/69 ← 10/23
DƯ : 680/69
Vậy sau pư chất còn dư là Al và số mol Al dư là 680/69 mol

Đổi 10kg = 10000g
Ta có: \(n_{CH_3COOH\left(LT\right)}=\dfrac{10000.5\%}{92\%}=\dfrac{12500}{23}\left(mol\right)\)
PTHH:
\(C_2H_5OH+O_2\xrightarrow[]{\text{men giấm}}CH_3COOH+H_2O\)
\(\dfrac{12500}{23}\)<---------------------\(\dfrac{12500}{23}\)
\(\Rightarrow m_{C_2H_5OH}=\dfrac{12500}{23}.46=25000\left(g\right)=25\left(kg\right)\)

a.\(V_{C_2H_5OH}=\dfrac{10.8}{100}=0,8ml\)
\(m_{C_2H_5OH}=0,8.0,8=1,6g\)
\(n_{C_2H_5OH}=\dfrac{1,6}{46}=0,034mol\)
\(C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\)
0,034 0,034 ( mol )
\(m_{CH_3COOH}=0,034.60.80\%=1,632g\)
b.\(m_{dd_{CH_3COOH}}=\dfrac{1,632}{5\%}=32,64g\)
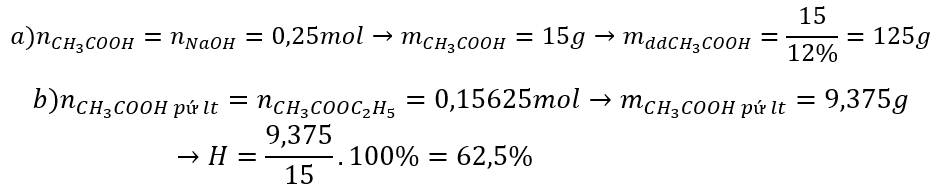

a, \(2K+2CH_3COOH\rightarrow2CH_3COOK+H_2\)
b, \(C_2H_5OH+O_2\underrightarrow{t^o,xt}CH_3COOH+H_2O\)
Ta có: \(n_{CH_3COOH}=\dfrac{12}{60}=0,2\left(mol\right)\)
Theo PT: \(n_{C_2H_5OH\left(LT\right)}=n_{CH_3COOH}=0,2\left(mol\right)\)
Mà: H = 80% \(\Rightarrow n_{C_2H_5OH\left(TT\right)}=\dfrac{0,2}{80\%}=0,25\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH\left(TT\right)}=0,25.46=11,5\left(g\right)\)
\(\Rightarrow V_{C_2H_5OH\left(TT\right)}=\dfrac{11,5}{0,8}=14,375\left(ml\right)\)
\(\Rightarrow V_{C_2H_5OH\left(10^o\right)}=\dfrac{14,375}{10}.100=143,75\left(ml\right)\)