Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

uiii em ơi, 2p mà viết và chụp xong luôn rồi à, nhanh thật, bái phục

\(n_{Fe}=\dfrac{m}{M}=\dfrac{2,8}{56}=0,05\left(mol\right)\)
\(n_{HCl}=\dfrac{m}{M}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
1 : 2 : 1 (mol)
0,05 : 0,4 (mol)
-Chuyển thành tỉ lệ: \(\dfrac{0,05}{1}< \dfrac{0,4}{2}\Rightarrow\) Fe phản ứng hết còn HCl dư.
-Theo PTHH: \(n_{H_2}=\dfrac{0,5.1}{1}=0,5\left(mol\right)\)
\(\Rightarrow V_{H_2}=n.22,4=0,5.22,4=11,2\left(l\right)\)
b) \(n_{Fe\left(cần\right)}=\dfrac{0,4.1}{2}=0,2\left(mol\right)\)
\(\Rightarrow n_{Fe\left(thêm\right)}=n_{Fe\left(cần\right)}-n_{Fe\left(tt\right)}=0,2-0,05=0,15\left(mol\right)\)
\(\Rightarrow m_{Fe\left(thêm\right)}=n.M=0,15.56=8,4\left(g\right)\)

a. 2Al + 3 \(CuSO_4\)→ 1 \(Al_2\left(SO_4\right)_3+3Cu\)
0.45 0,3375 (mol)
⇔0,225.2 0,1125.3 (mol)
0,3375 -----→ \(\dfrac{0,3375.1}{3}\)=0,1125 (mol)
(lấy số mol lớn - số mol bé ➙ số mol dư)
b. \(n_{Al}\)= \(\dfrac{12,15}{27}\)=0,45 (mol)
\(n_{CuSO_4}\)= \(\dfrac{54}{64+32+16.4}\)=0,3375(mol)
➝ \(n_{Al}\)dư= 0,1125 (mol)
⇒\(m_{Al_{dư}}\)= 0,1125.27=3.0375(gam)
⇒\(m_{Al_2\left(SO_4\right)_3}\)= 0,1125. \(\left[27.2+2\left(32+16.4\right)\right]\)=27,675(gam)

1.
a, \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right);n_{HCl}=\dfrac{18,25}{36,5}=0,5\left(mol\right)\)
PTHH: Mg + 2HCl → MgCl2 + H2
Mol: 0,15 0,3
b, Ta có: \(\dfrac{0,15}{1}< \dfrac{0,5}{2}\) ⇒ Mg pứ hết, HCl dư
\(m_{HCldư}=\left(0,5-0,3\right).36,5=7,3\left(g\right)\)
c, \(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
2.
a, \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
PTHH: 4P + 5O2 ---to→ 2P2O5
Mol: 0,2 0,25 0,1
b, \(V_{O_2}=0,25.22,4=5,6\left(l\right)\)
c, \(m_{P_2O_5}=0,1.142=14,2\left(g\right)\)
d, \(V_{kk}=5,6.5=28\left(l\right)\)

\(n_{Fe}=\dfrac{5,6}{56}=0,1mol\)
\(n_{HCl}=\dfrac{14,6}{36,5}=0,4mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,1 < 0,4 ( mol )
0,1 0,2 0,1 0,1 ( mol )
Chất dư là HCl
\(m_{HCl\left(dư\right)}=\left(0,4-0,2\right).36,5=7,3g\)
\(V_{H_2}=0,1.22,4=2,24l\)
\(m_{FeCl_2}=0,1.127=12,7g\)
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\\
n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\\
pthh:Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
\(LTL:\dfrac{0,1}{1}< \dfrac{0,4}{1}\)
=> H2SO4 d
\(n_{H_2SO_4\left(pu\right)}=n_{Fe}=0,1\left(mol\right)\\
m_{H_2SO_4\left(d\right)}=\left(0,4-0,1\right).98=29,4g\)
\(n_{H_2}=n_{FeSO_4}=n_{Fe}=0,1\left(mol\right)\)
\(V_{H_2}=0,1.22,4=2,24l\\
m_{FeSO_4}=0,1.152=15,2g\)

a) \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
\(n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
Xét tỉ lệ: \(\dfrac{0,1}{2}< \dfrac{0,4}{6}\) => Al hết, HCl dư
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,1--->0,3------>0,1---->0,15
=> mHCl = (0,4 - 0,3).36,5 = 3,65 (g)
b) VH2 = 0,15.22,4 = 3,36 (l)
\(n_{Al}=\dfrac{m_{Al}}{M_{Al}}=\dfrac{2,7}{27}=0,1mol\)
\(n_{HCl}=\dfrac{m_{HCl}}{M_{HCl}}=\dfrac{14,6}{36,5}=0,4mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,1 < 0,4 ( mol )
0,1 0,3 0,15 ( mol )
a. Chất còn dư là HCl
\(m_{HCl}=n_{HCl}.M_{HCl}=\left(0,4-0,3\right).36,5=3,65g\)
\(V_{H_2}=n_{H_2}.22,4=0,15.22,4=3,36l\)
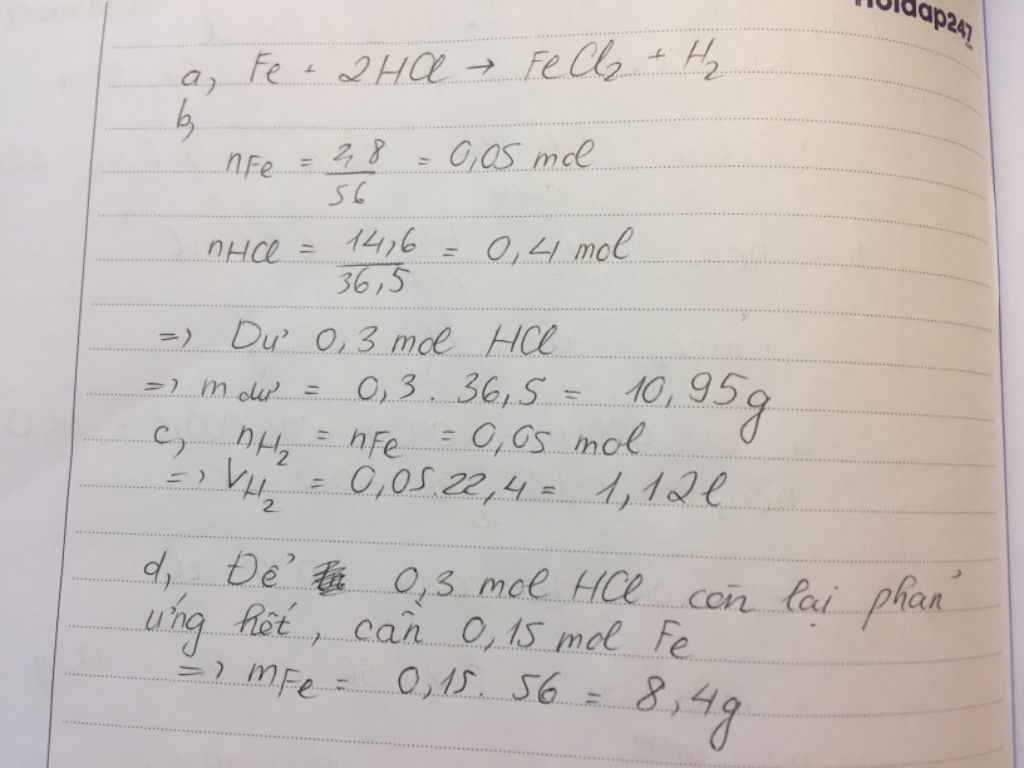


\(n_{Fe}=\dfrac{2,8}{56}=0,05\left(mol\right)\\ n_{HCl}=\dfrac{14,6}{36,5}=0,4\left(mol\right)\)
PTHH: Fe + 2HCl ---> FeCl2 + H2
LTL: \(0,05< \dfrac{0,4}{2}\rightarrow\) HCl dư
Theo pthh: \(\left\{{}\begin{matrix}n_{H_2}=n_{Fe}=0,05\left(mol\right)\\n_{HCl\left(pư\right)}=2n_{Fe}=0,05.2=0,1\left(mol\right)\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}V_{H_2}=0,05.22,4=1,12\left(l\right)\\m_{HCl\left(dư\right)}=\left(0,4-0,1\right).36,5=10,95\left(g\right)\end{matrix}\right.\)
Theo pthh: \(n_{Fe\left(thêm\right)}=\dfrac{1}{2}n_{HCl\left(dư\right)}=\dfrac{1}{2}.\left(0,4-0,1\right)=0,15\left(mol\right)\)
\(\rightarrow m_{Fe\left(thêm\right)}=0,16.56=8,4\left(g\right)\)