Đốt cháy 2,4g Mg trong o2 thủ được Mgo tính mo2,co2,mMgO
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(PTHH:2Mg+O_2\rightarrow^{t^o}2MgO\\ \text{Bảo toàn KL: }m_{Mg}+m_{O_2}=m_{MgO}\\ \Rightarrow\text{Chọn B}\)

\(n_{Mg}=\dfrac{1,8}{24}=0,075(mol)\\ 2Mg+O_2\xrightarrow{t^o}2MgO\\ \Rightarrow n_{MgO}=0,075(mol)\\ \Rightarrow m_{MgO}=0,075.40=3(g)\)

\(n_{Mg}=\dfrac{7,2}{24}=0,3\left(mol\right)\\ 2Mg+O_2\rightarrow\left(t^o\right)2MgO\\ n_{O_2}=\dfrac{0,3}{2}=0,15\left(mol\right)\\ n_{MgO}=n_{Mg}=0,3\left(mol\right)\\ a,V_{O_2\left(đktc\right)}=0,15.22,4=3,36\left(l\right)\\ b,m_{MgO}=0,3.40=12\left(g\right)\)

Sửa đề: 36 (g) → 3,6 (g)
Ta có: \(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
PT: \(2Mg+O_2\underrightarrow{t^o}2MgO\)
Theo PT: \(n_{MgO\left(LT\right)}=n_{Mg}=0,1\left(mol\right)\Rightarrow m_{MgO\left(LT\right)}=0,1.40=4\left(g\right)\)
\(\Rightarrow H=\dfrac{3,6}{4}.100\%=90\%\)

Câu 19
\(n_{CO_2}=\dfrac{22}{44}=0,5\left(mol\right)\)
=> VCO2 = 0,5.22,4 = 11,2 (l)
\(n_{O_2}=\dfrac{8}{32}=0,25\left(mol\right)\)
=> VO2 = 0,25.22,4 = 5,6 (l)
\(n_{N_2}=\dfrac{2,8}{28}=0,1\left(mol\right)\)
=> VN2 = 0,1.22,4 = 2,24 (l)
Câu 20
a) 2Mg + O2 --to--> 2MgO
b) \(n_{Mg}=\dfrac{24}{24}=1\left(mol\right)\)
2Mg + O2 --to--> 2MgO
_1--->0,5-------->1
=> mMgO = 1.40 = 40(g)
=> VO2 = 0,5.22,4 = 11,2 (l)

a) 2Mg + O2 --to--> 2MgO
b) \(n_{Mg}=\dfrac{18}{24}=0,75\left(mol\right)\)
=> nMgO = 0,75 (mol)
=> mMgO = 0,75.40 = 30(g)
c) nO2 = 0,375 (mol)
=> VO2 = 0,375.24,79 = 9,29625 (l)

\(n_{Mg}=0,15\left(mol\right)\)
\(2Mg+O_2\underrightarrow{t^o}2MgO\)
0,15-------------0,15 (mol)
\(m_{MgO}=0,15.40=6\left(g\right)\)

Bài 2:
a) 2Mg + O2 --to--> 2MgO
b) \(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\)
PTHH: 2Mg + O2 --to--> 2MgO
_______0,2->0,1------>0,2
=> VO2 = \(\dfrac{0,1.0,082.\left(273+25\right)}{0,99}=2,468\left(l\right)\)
c) mMgO = 0,2.40 = 8(g)
Bài 3
a) Theo ĐLBTKL: mMg + mO2 = mMgO (1)
b) (1) => mMgO = 2,4 + 1,6 = 4(g)
c) \(nO_2=\dfrac{1,6}{32}=0,05\left(mol\right)\)
=> Số phân tử O2 = 0,05.6.1023 = 0,3.1023

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ a,2Mg+O_2\rightarrow\left(t^o\right)2MgO\\ n_{O_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ V_{O_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ b,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\\ n_{KClO_3}=\dfrac{0,05.2}{3}=\dfrac{1}{30}\left(mol\right)\\ \Rightarrow m_{KClO_3}=\dfrac{122,5}{30}=\dfrac{49}{12}\left(g\right)\)
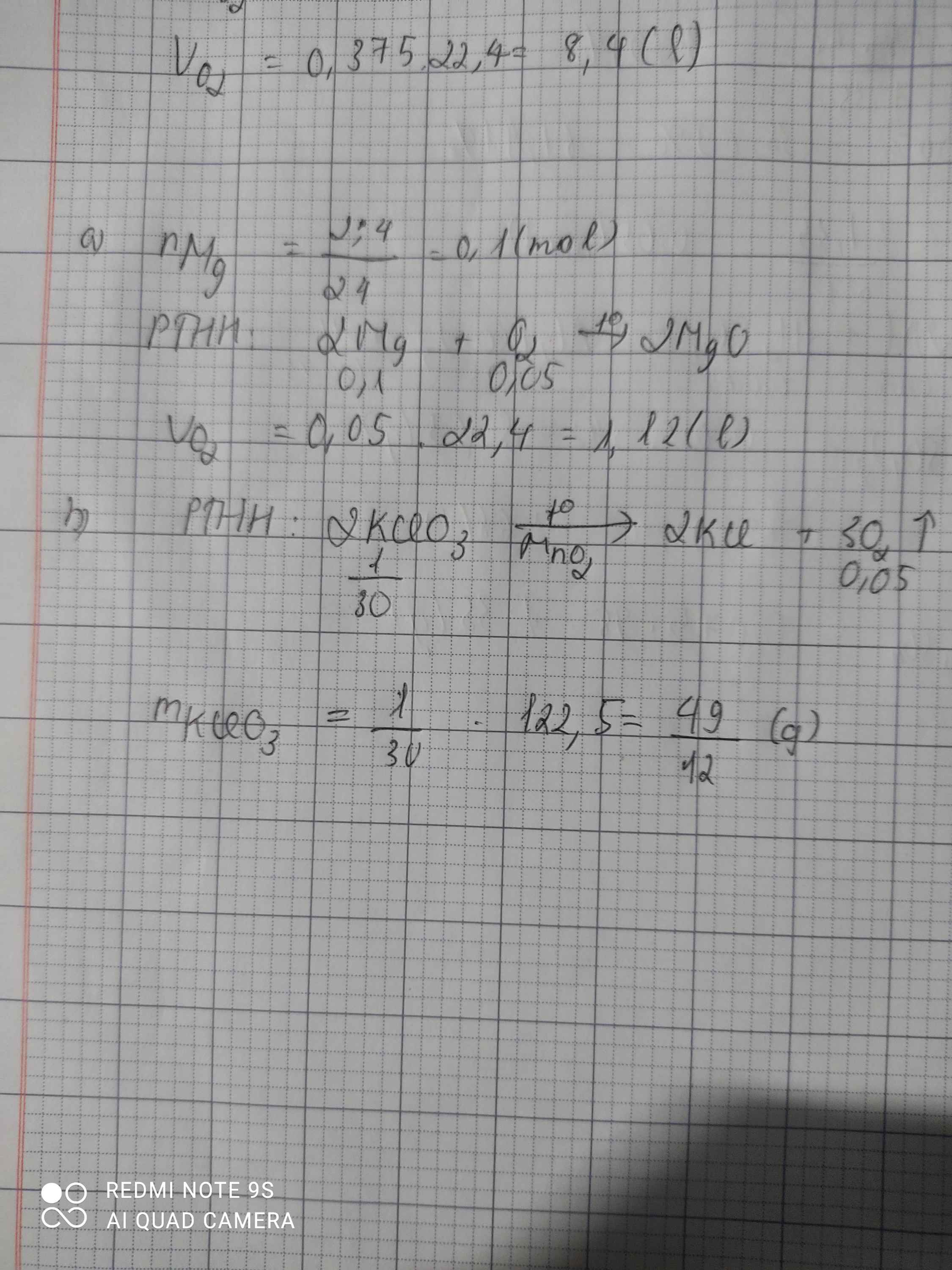
\(n_{Mg}=\dfrac{2,4}{24}=0,1mol\)
\(2Mg+O_2\rightarrow\left(t^o\right)2MgO\)
0,1 0,05 0,1 ( mol )
\(m_{O_2}=0,05.32=1,6g\)
\(V_{O_2}=0,05.22,4=1,12l\)
\(m_{MgO}=0,1.40=4g\)
2Mg+O2-to>2MgO
0,1-----0,05-------0,1
n Mg=\(\dfrac{2,4}{24}\)=0,1 mol
=>m O2=0,05.22,4=1,12l
=>m MgO=0,1.40=4g