mọi người ơi giải chi tiết giúp em nha
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Bài 2:
\(a,n_{H_2}=\dfrac{1,12}{22,4}=0,05(mol)\\ PTHH:Mg+2HCl\to MgCl_2+H_2\\ MgO+2HCl\to MgCl_2+H_2O\\ \Rightarrow n_{Mg}=0,05(mol)\\ \Rightarrow m_{Mg}=24.0,05=1,2(g)\\ \Rightarrow m_{MgO}=9,2-1,2=8(g) b,\%_{Mg}=\dfrac{1,2}{9,2}.100\%=13,04\%\\ \Rightarrow \%_{MgO}=100\%-13,04\%=86,96\%\\ c,n_{MgO}=\dfrac{8}{40}=0,2(mol)\\ \Rightarrow \Sigma n_{HCl}=2n_{Mg}+2n_{MgO}=0,5(mol)\\ \Rightarrow \Sigma m_{HCl}=0,5.36,5=18,25(g)\\ \Rightarrow m_{dd_{HCl}}=\dfrac{18,25}{14,6\%}=125(g)\)

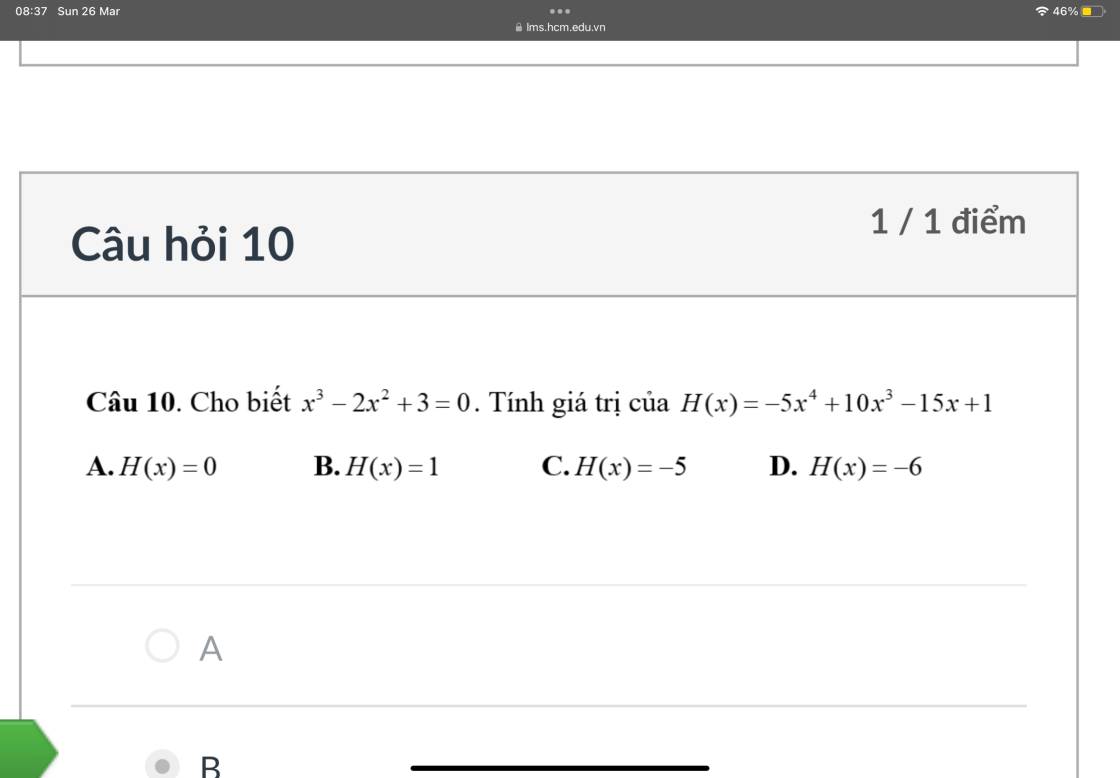
10.
\(H\left(x\right)=-5x^4+10x^3-15x+1\)
\(=-5x\left(x^3-2x^2+3\right)+1\)
\(=-5x.0+1\)
\(=1\)
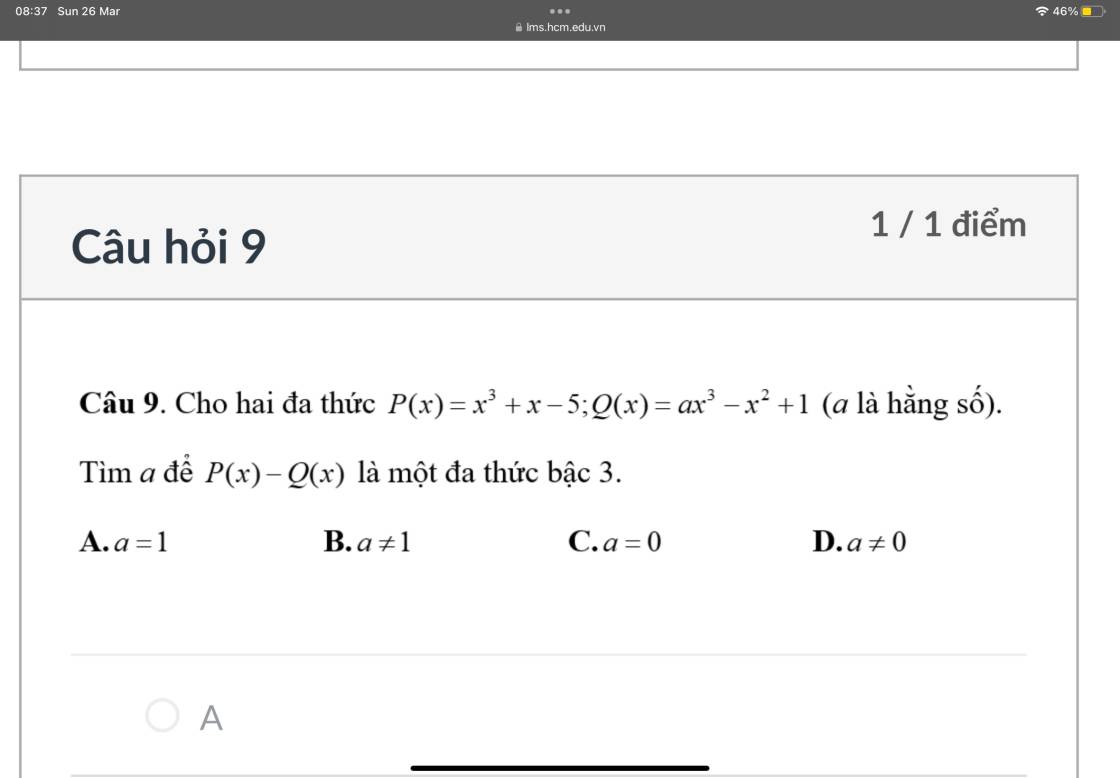
9.
\(P\left(x\right)-Q\left(x\right)=\left(1-a\right)x^3+x^2+x-6\)
\(P\left(x\right)-Q\left(x\right)\) là đa thức bậc 3 khi và chỉ khi \(1-a\ne0\)
\(\Rightarrow a\ne1\)

Ta có
\(a^2+1=a^2+ab+bc+ca=a\left(a+b\right)+c\left(a+b\right)=\left(a+b\right).\left(a+c\right)\\ Cmtt:b^2+1=\left(b+a\right).\left(b+c\right)\\ c^2+1=\left(c+a\right).\left(c+b\right)\)
Nên
\(\dfrac{b-c}{a^2+1}+\dfrac{c-a}{b^2+1}+\dfrac{a-b}{c^2+1}\\ =\dfrac{\left(b-c\right)}{\left(a+b\right)\left(a+c\right)}+\dfrac{\left(c-a\right)}{\left(b+c\right)\left(b+a\right)}+\dfrac{\left(a-b\right)}{\left(c+a\right)\left(c+b\right)}\\ =\dfrac{\left(b-c\right)\left(b+c\right)+\left(c-a\right)\left(c+a\right)+\left(a-b\right)\left(a+b\right)}{\left(a+b\right)\left(b+c\right)\left(c+a\right)}\\ =\dfrac{b^2-c^2+c^2-a^2+a^2-b^2}{\left(a+b\right)\left(b+c\right)\left(c+a\right)}\\ =0\)
\(\dfrac{b-c}{a^2+1}+\dfrac{c-a}{b^2+1}+\dfrac{a-b}{c^2+1}\)
\(=\dfrac{b-c}{a^2+ab+bc+ac}+\dfrac{c-a}{b^2+ab+bc+ca}+\dfrac{a-b}{c^2+ab+bc+ca}\)
\(=\dfrac{b-c}{a\left(a+b\right)+c\left(a+b\right)}+\dfrac{c-a}{b\left(a+b\right)+c\left(a+b\right)}+\dfrac{a-b}{c\left(c+a\right)+b\left(a+c\right)}\)
\(=\dfrac{b-c}{\left(a+c\right)\left(a+b\right)}+\dfrac{c-a}{\left(b+c\right)\left(a+b\right)}+\dfrac{a-b}{\left(b+c\right)\left(a+c\right)}\)
\(=\dfrac{\left(b-c\right)\left(b+c\right)+\left(c-a\right)\left(a+c\right)+\left(a-b\right)\left(a+b\right)}{\left(a+c\right)\left(a+b\right)\left(b+c\right)}\)
\(=\dfrac{b^2-c^2+c^2-a^2+a^2-b^2}{\left(a+b\right)\left(b+c\right)\left(c+a\right)}=0\)

Ta có:
(2 - 3x)(x + 8) = (3x - 2)(3 - 5x)
⇔ (2 - 3x)(x + 8) - (3x - 2)(3 - 5x) = 0
⇔ (2 - 3x)(x + 8) + (2 - 3x)(3 - 5x) = 0
⇔ (2 - 3x)(x + 8 + 3 - 5x) = 0
⇔ (2 - 3x)(11 - 4x) = 0
⇔ 2 - 3x = 0 hay 11 - 4x = 0
⇔ 2 = 3x hay 11 = 4x
⇔ x = \(\dfrac{2}{3}\) hay x = \(\dfrac{11}{4}\)
Vậy tập nghiệm của pt S = \(\left\{\dfrac{2}{3};\dfrac{11}{4}\right\}\)
<=> (2-3x ) (x+8) + (2-3x ) (3-5x)=0
<=> (2-3x ) ( x+8 + 3-5x ) =0
<=> (2-3x ) ( 11 - 4x ) = 0
=> 2-3x =0 hoặc 11-4x =0
3x = 2 4x =11
x = 2/3 x = 11/4

pt 2CH3COOH+Mg→(CH3COO)2Mg +H2
n(CH3COO)2Mg =1,42/142=0,1 mol
theo pt nCH3COOH =2n(CH3COO)2Mg =0,2 mol
suy ra CM=0,2 /0,5=0.4 mol/l
theo pt nH2 =n(CH3COO)2Mg =0,1 mol
suy ra VH2 =2,24l
KOH+CH3COOH->CH3COOK+H2O
0,2------0,2
=>VKOH=\(\dfrac{0,2}{0,5}\)=0,4l=400ml

\(1,4x\left(1-x\right)-8=1-\left(4x^2+3\right)\\ \Leftrightarrow4x-4x^2-8=1-4x^2-3\\ \Leftrightarrow4x-4x^2-8-1+4x^2+3=0\\ \Leftrightarrow4x-6=0\\ \Leftrightarrow x=\dfrac{3}{2}\)
\(2,\left(2-3x\right)\left(x+11\right)=\left(3x-2\right)\left(2-5x\right)\\ \Leftrightarrow\left(2-3x\right)\left(x+11\right)-\left(2-3x\right)\left(5x-2\right)=0\\ \Leftrightarrow\left(2-3x\right)\left(x+11-5x+2\right)=0\\ \Leftrightarrow\left(2-3x\right)\left(-4x+13\right)=0\\ \Leftrightarrow\left[{}\begin{matrix}x=\dfrac{2}{3}\\x=\dfrac{13}{4}\end{matrix}\right.\)

1) 2NH3 + 3Cl2 --> N2 + 6HCl
Chất khử: NH3, chất oxh: Cl2
| 2N-3 -6e--> N20 | x1 |
| Cl20 +2e--> 2Cl- | x3 |
2) 4NH3 + 5O2 --> 4NO + 6H2O
Chất khử: NH3, chất oxh: O2
| N-3 -5e--> N+2 | x4 |
| O20 +4e--> 2O-2 | x5 |
3) 8Al + 3Fe3O4 --> 4Al2O3 + 9Fe
Chất khử: Al, chất oxh: Fe3O4
| \(2Al^0-6e\rightarrow Al_2^{+3}\) | x4 |
| \(Fe_3^{+\dfrac{8}{3}}+8e\rightarrow3Fe^0\) | x3 |
4) MnO2 + 4HCl --> MnCl2 + Cl2 + 2H2O
Chất khử: HCl, chất oxh: MnO2
| Mn+4 +2e-->Mn+2 | x1 |
| 2Cl- -2e--> Cl20 | x1 |
5) 16HCl + 2KMnO4 --> 2MnCl2 + 2KCl + 5Cl2 + 8H2O
Chất khử: HCl, chất oxh: KMnO4
| 2Cl- -2e--> Cl20 | x5 |
| Mn+7 +5e--> Mn+2 | x2 |
6) 3Cu + 8HNO3 --> 3Cu(NO3)2 + 2NO + 4H2O
Chất khử: Cu, chất oxh: HNO3
| Cu0-2e--> Cu+2 | x3 |
| N+5+3e--> N+2 | x2 |
7) 4Zn + 10HNO3 --> 4Zn(NO3)2 + NH4NO3 + 3H2O
Chất khử: Zn, chất oxh: HNO3
| Zn0-2e-->Zn+2 | x4 |
| N+5 +8e--> N-3 | x1 |
8) 8Al + 30HNO3 --> 8Al(NO3)3 + 3N2O + 15H2O
Chất khử: Al, chất oxh: HNO3
| Al0-3e--> Al+3 | x8 |
| 2N+5 +8e--> N2+1 | x3 |
9) 2Al + 6H2SO4 --> Al2(SO4)3 + 3SO2 + 6H2O
Chất khử: Al, chất oxh: H2SO4
| Al0-6e--> Al2+3 | x1 |
| S+6+2e--> S+4 | x3 |
10) 2KMnO4 + 10FeSO4 + 8H2SO4 --> 2MnSO4 + 5Fe2(SO4)3 + K2SO4 + 8H2O
Chất khử: FeSO4, chất oxh: KMnO4
| 2Fe+2 -2e--> Fe2+3 | x5 |
| Mn+7 +5e--> Mn+2 | x2 |