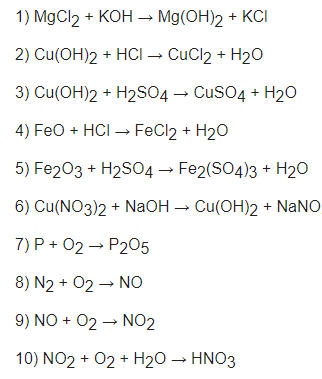
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


`#1194`
Qtr sx thép:
- Nguyên liệu: gang, sắt, thép phế liệu, khí oxygen
- Cách thực hiện:
+ Khí oxy được thổi từ dưới lò lên để đốt cháy tạp chất trong gang
+ Các oxide tạo thành ở dạng khí \(\left(\text{CO}_2;\text{ }\text{ SO}_2;...\right)\) sẽ thoát ra theo khí thải, các oxide dạng rắn \(\left(\text{SiO}_2;\text{ MnO}_2;...\right)\) sẽ tạo ra xỉ nhẹ, nổi lên trên thép nóng.
- Xỉ được tách ra để thu lấy thép.

a, Khối lượng \(m_{CaNa_2}=n.M=36.\left(40+23.2\right)=3096\left(g\right)\)
b, Khối lượng \(m_{SCO_2}=n.M=56.\left(32+12+16.2\right)=4256\left(g\right)\)
mcaNa=36+40+23x2=132 (gam)
mSCo= 56+32+16x2=208 (gam)

- Huyền phù là một hỗn hợp không đồng nhất gồm các hạt chất rắn phân tán lơ lửng trong môi trường chất lỏng.
Huyền phù" là một thuật ngữ trong hóa học, không phải là một cá nhân. Nó đề cập đến một hệ thống phân tán không đồng nhất của các hạt rắn trong một chất lỏng hoặc khí. Khi các hạt rắn không tan hoặc tan không đều trong chất lỏng, chúng sẽ tạo thành lớp rắn lỏng lẻo, gọi là "huyền phù".


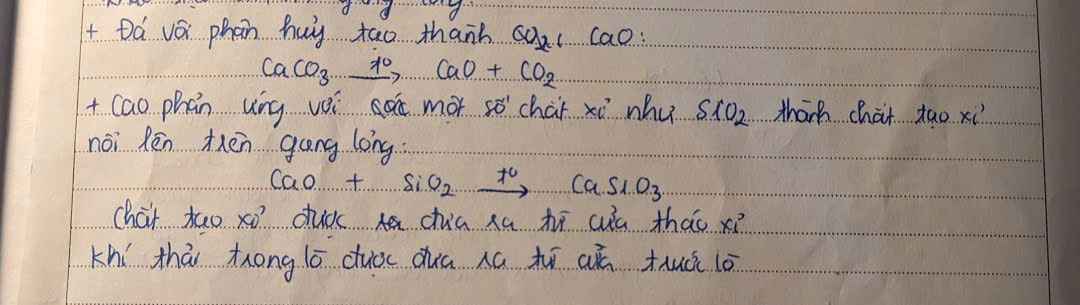
1) MgCL2 + 2KOH ==> Mg(OH)2 + 2KCl
2) Cu(OH)2 + 2HCl ==> CuCl2 + 2H2O
3) CuSO4 + H2SO4 ==> CuSO4 + 2H2O
4) FeO + 2HCl ==> FeCL2 + H2O
5) Fe2O3 + 3H2SO4 ==> Fe2(SO4)3 + 3H2O
6) Cu(NO3)2 + 2NaOH ==> Cu(OH)2 + 2NaNO3
7) 4P + 5O2 ==> 2P2O5
8) N2 + O2 ==> 2NO
9) 2NO + O2 ==> 2NO2
10) 4NO2 + 3O2 + 2H2O ==> 4HNO3