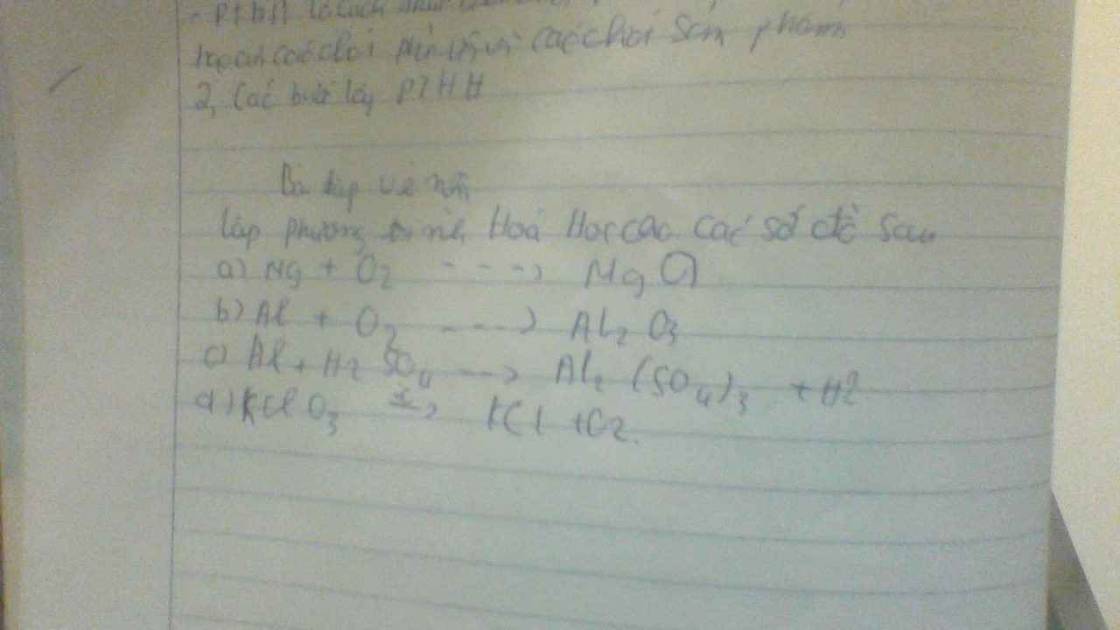Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



a, Có: \(M_X=40.10=400\left(g/mol\right)\)
\(\Rightarrow2M_M+96.3=400\Rightarrow M_M=56\left(g/mol\right)\)
→ M là Fe.
b, Ta có: \(\dfrac{2M_M}{2M_M+96.3}=0,1579\) \(\Rightarrow M_M=27\left(g/mol\right)\)
→ M là Al.
ngfngfha qbadkcjvwerertwer ư4bt h ưeascfgeq g ưhrewtherth3tr t6j etyk5wergqerg ẻgwergw ẻgerg8erg8uewrgwjberhg bbẻgihewrgweirgewrgwerogewr euo 9guergwerhuigher
gewrg\ewrg\ưerg\ưerger
gewr
g
ẻg
ẻg
ư
ẻg
ửhwer hửherh
ẻ
e
rh
ử
hẻutj
rthkm, uku
jt7
ỵt4u
6k7o
rrkjkt7
rkrj4
tug
jn5y
ụ46etyk
ẹmt
rfdu
5j5jetrf
etet
gj
jjgge
jgetetge
jg
j

\(1.\\ a.Fe+2HCl\rightarrow FeCl_2+H_2\\ FeCO_3+2HCl\rightarrow FeCl_2+H_2O+CO_2\)
\(n_{\uparrow}=\dfrac{4,48}{22,4}=0,2mol\\ n_{Fe}=a;n_{FeCO_3}=b\\ \Rightarrow\left\{{}\begin{matrix}56a+116b=28,4:2=14,2\\a+b=0,2\end{matrix}\right.\\ \Rightarrow a=0,15;b=0,05\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,15 0,3 0,15 0,15
\(FeCO_3+2HCl\rightarrow FeCl_2+CO_2+H_2O\)
0,05 0,1 0,05 0,05 0,05
\(n_{NaOH}=0,2.0,3=0,06mol\)
\(T=\dfrac{0,06}{0,05}=1,2\)
⇒Tạo \(Na_2CO_3\) và \(NaHCO_3\)
\(2NaOH+CO_2\rightarrow Na_2CO_3+H_2O\)
\(NaOH+CO_2\rightarrow NaHCO_3\)
\(m_{Fe}=0,15.56.2=16,8g\\ m_{FeCO_3}=0,05.116.2=11,6g\)
\(b.n_{Na_2CO_3}=a;n_{NaHCO_3}=b\\ \Rightarrow\left\{{}\begin{matrix}2a+b=0,06\\a+b=0,05\end{matrix}\right.\\ \Rightarrow a=0,01;b=0,04\)
\(C_{M_{Na_2CO_3}}=\dfrac{0,01}{0,02}=0,5M\\ C_{M_{NaHCO_3}}=\dfrac{0,04}{0,02}=2M\\ m_{rắn}=0,01.106+0,04.84=4,42g\)
\(c.n_{HCl}=a\)
\(C_{\%HCl.dư}=\dfrac{\left(a-0,3-0,1\right).36,5}{\dfrac{36,5a}{20}\cdot100+28,4:2-0,05.44-0,15.2}\cdot100=11,53\%\\ \Rightarrow a\approx1,03mol\)
\(m_{dd}=\dfrac{1,03.36,5}{20}\cdot100+28,4:2-0,05.44-0,15.2=199,675g\)
\(C_{\%FeCl_2}=\dfrac{\left(0,15+0,05\right)127}{199,675}\cdot100\approx12,72\%\)
\(2.\)
Dd E là gì vậy bạn?

\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo pt: \(n_{Al}=\dfrac{2}{3}n_{H_2}=0,2mol\)

\(n_{Fe}=\dfrac{6,72}{56}=0,12mol\\ 2Fe+O_2\xrightarrow[]{t^0}2FeO\)
0,12 0,06 0,12
\(m_{FeO}=0,12.72=8,64g\\ V_{O_2}=0,06.24,79=1,4874l\)

a, \(2MgO+O_2\underrightarrow{t^o}2MgO\)
b, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
c, \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
d, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(a,2Mg+O_2\rightarrow\left(t^o\right)2MgO\)
\(b,4Al+3O_2\rightarrow2Al_2O_3\)
\(c,2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
\(d,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\)