Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_P=\dfrac{m}{M}=\dfrac{6,2}{31}=0,2\left(mol\right)\)
\(PTHH:4P+5O_2-^{t^o}>2P_2O_5\)
tỉ lệ 4 : 5 : 2
n(mol) 0,2---->0,25---->0,1
`V(O_2)=nxx24,79=0,25xx24,79=6,1975(l)`
`V(kk)=6,1975:1/5=30,9875(l)`

Ta có: \(n_P=\dfrac{12,4}{31}=0,4\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
_____0,4____0,5_____0,2 (mol)
a, \(m_{P_2O_5}=0,2.142=28,4\left(g\right)\)
b, \(V_{O_2}=0,5.22,4=11,2\left(l\right)\)

a.\(n_P=\dfrac{1,55}{31}=0,05\left(mol\right)\)
PTHH : 4P + 5O2 -> 2P2O5
0,05 0,0625 0,025
\(V_{O_2}=0,0625.22,4=1,4\left(l\right)\)
b. \(m_{P_2O_5}=0,025.142=3,55\left(g\right)\)

\(n_{Cu}=\dfrac{2,56}{64}=0,04mol\)
\(2Cu+O_2\underrightarrow{t^o}2CuO\)
0,04 0,02 0,04
\(V_{O_2}=0,02\cdot22,4=4,48l\)
\(m_{CuO}=0,04\cdot80=3,2g\)
nCu = 2,56 : 64 =0,04 (mol)
pthh : 2Cu + O2 -t--> 2CuO
0,04->0,02----->0,04 (mol)
VO2 = 0,02 .22,4 =0,448 (l)
mCuO = 0,04 . 80 =3,2 (g)

4P+5O2-to>2P2O5
0,05--0,0625------0,025 mol
n P=\(\dfrac{1,55}{31}\)=0,05 mol
=>VO2=0,0625.22,4=1,4l
=> mP2O5=0,025.142=3,55g

\(4P+5O_2\xrightarrow{t^o}2P_2O_5\\ n_P=\dfrac{6,2}{31}=0,2(mol)\\ \Rightarrow n_{P_2O_5}=0,1(mol);n_{O_2}=0,25(mol)\\ \Rightarrow m_{P_2O_5}=0,1.142=14,2(g);V_{O_2}=0,25.22,4=5,6(l)\)

\(n_{KClO_3}=\dfrac{12,25}{122,5}=0,1\left(mol\right)\)
PTHH: 2KClO3 -to, MnO2-> 2KCl + 3O2
0,1--------------------->0,1--->0,15
=> \(\left\{{}\begin{matrix}V_{O_2}=0,15.24,79=3,7185\left(l\right)\\m_{KCl}=0,1.74,5=7,45\left(g\right)\end{matrix}\right.\)
\(n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
PTHH: 4P + 5O2 --to--> 2P2O5
LTL: \(\dfrac{0,1}{4}< \dfrac{0,15}{5}\) => P có cháy hết

a) \(n_{Fe}=\dfrac{33,6}{56}=0,6\left(mol\right)\)
PTHH: \(3Fe+2O_2\xrightarrow[]{t^o}Fe_3O_4\)
0,6--->0,4------->0,2 (mol)
=> \(m_{Fe_3O_4}=0,2.232=46,4\left(g\right)\)
b) \(V_{O_2\left(\text{đ}kc\right)}=0,4.24,79=9,916\left(l\right)\)
c) PTHH: \(2KClO_3\xrightarrow[]{t^o}2KCl+3O_2\)
\(\dfrac{4}{15}\)<-------------------0,4 (mol)
=> \(m_{KClO_3}=\dfrac{4}{15}.122,5=\dfrac{98}{3}\left(g\right)\)

nO2 = 2,479/24,79 = 0,1 (mol)
PTHH: 4P + 5O2 -> (t°) 2P2O5
Mol: 0,08 <--- 0,1 ---> 0,04
mP2O5 = 0,05 . 142 = 5,68 (g)
\(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
\(nO_2=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
\(\Rightarrow nP_2O_5=\dfrac{2}{5}.nO_2=\dfrac{2}{5}.0,1=0,04\left(mol\right)\)
\(mP_2O_5=0,04.142=5,68\left(g\right)\)

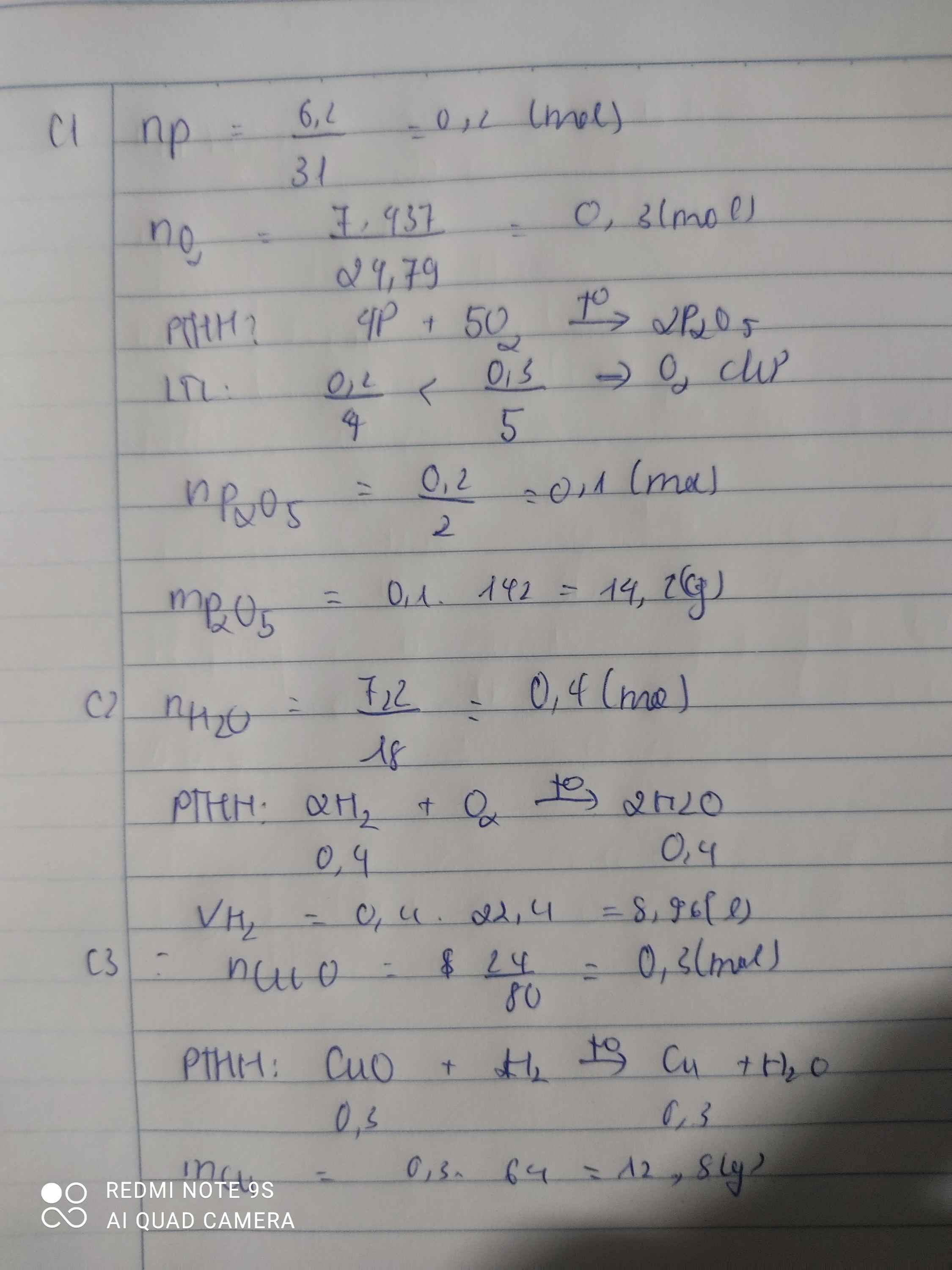

Ta có: \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
____0,2__0,25_____0,1 (mol)
⇒ VO2 = 0,25.24,79 = 6,1975 (l)
mP2O5 = 0,1.142 = 14,2 (g)