Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(MnO_2+4HCl\underrightarrow{t^o}MnCl_2+Cl_2+2H_2O\)
\(Cl_2+H_2\underrightarrow{as}2HCl\)
\(NaOH+HCl\rightarrow NaCl+H_2O\)
\(2NaCl+2H_2O\underrightarrow{đpcmn}2NaOH+H_2+Cl_2\)
\(Cl_2+2NaBr\rightarrow2NaCl+Br_2\)
\(2NaI+Br_2\rightarrow2NaBr+I_2\)
\(2MnO_2+4HCl\rightarrow2MnCl_2+Cl_2+2H_2O\)
\(Cl_2+H_2\rightarrow2HCl\)
\(HCl+NaOH\rightarrow NaCl+H_2O\)
\(2NaCl\rightarrow2Na+Cl_2\)
\(Cl_2+2HBr\rightarrow2HCl+Br_2\)

$Cl_2 + H_2O \rightleftharpoons HCl + HClO \\ Cl_2 + 2NaOH \to NaCl + NaClO + H_2O\\ MnO_2 + 4HCl \xrightarrow{t^o} MnCl_2 + Cl_2 + 2H_2O \\ 2KMnO_4 + 16HCl \to 2MnCl_2 + 2KCl + 5Cl_2 + 8H_2O \\ 2NaCl + 2H_2O \xrightarrow{điện\ phân\ dung\ dịch,có\ màng\ ngăn} 2NaOH + H_2 + Cl_2$
\(2Cl_2+2H_2O\rightarrow4HCl+O_2\)
\(2Cl_2+4NaOH\rightarrow4NaCl+2H_2O\)
\(2NaCl+2H_2O\rightarrow2NaOH+Cl_2+H_2\)

a, Cl2 + 2Na -> (t°) 2NaCl
2NaCl + H2SO4 -> Na2SO4 + 2HCl
4HCl + MnO2 -> MnCl2 + 2H2O + Cl2
Ca + Cl2 -> (t°) CaCl2
b, 4HCl + MnO2 -> MnCl2 + 2H2O + Cl2
2Fe + 3Cl2 -> (t°) 2FeCl3
FeCl3 + 3NaOH -> Fe(OH)3 + 3NaCl
2NaCl + H2SO4 -> Na2SO4 + 2HCl
c, MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2
3Cl2 + 6KOH (đặc nóng) -> 5KCl + KClO3 + 3H2O
2KCl + H2SO4 -> K2SO4 + 2HCl
4HCl + MnO2 -> MnCl2 + 2H2O + Cl2
2Cl2 + 2Ca(OH)2 -> CaCl2 + Ca(OCl)2 + 2H2O
d, 16HCl + 2KMnO4 -> 5Cl2 + 8H2O + 2KCl + 2MnCl2
3Cl2 + 6KOH (đặc nóng) -> 5KCl + KClO3 + 3H2O
2KCl -> (đpnc) 2K + Cl2
Cl2 + 2NaBr -> 2NaCl + Br2
Br2 + 2NaI -> 2NaBr + I2

\(KMnO_4+HCl_{\left(đ\right)}\rightarrow\left(t^o\right)KCl+MnCl_2+Cl_2\uparrow+H_2O\)
Quá trình trao đổi e:
\(\times2\) \(Mn^{+7}+5e\rightarrow Mn^{+2}\)
\(\times5\) \(2Cl^{-1}-2e\rightarrow Cl^0_2\)
\(\Rightarrow2KMnO_4+16HCl_{\left(đ\right)}\rightarrow\left(t^o\right)2KCl+2MnCl_2+5Cl_2\uparrow+8H_2O\)

4HCl+MnO2-->MnCl2+2H2O+Cl2
3Cl2+2Fe-->2FeCl3
FeCl3+3NaOH-->3NaCl+Fe(OH)3
2NaCl+H2SO4-->Na2SO4+2HCl
2HCl+Cuo-->CuCl2+H2O
CuCl2+2AgNO3-->2AgCl+Cu(NO3)2

 3)
3)
3Cl2+6KOH→3H2O+5KCl+KClO3
2KClO3→2KCl+3O2
2KCl+H2SO4→K2SO4+2HCl
16HCl+2KMnO4→5Cl2+8H2O+2KCl+2MnCl2
2Ca(OH)2+2Cl2→2H2O+CaCl2+Ca(ClO)2 e)a. 2KMnO4 + 16HCl (đ) -> 2KCl + 2MnCl2 + 5Cl2 + 8H2O (Đ.C Cl2)
Cl2 + KOH -> KCl + KClO3 + H2O
2KClO3 -> 2KCl + 3O2 (đ/c khí O2 lớp 8)
2KCl -> 2K + Cl2
Cl2 + H2O ->HCl + HClO
2HCl + Fe -> FeCl2 + H2
2FeCl2 + Cl2 -> 2FeCl3
FeCl3 + NaOH -> Fe(OH)3 + NaCl

Đáp án A
Sử dụng yếu tố diện tích tiếp xúc, TN1 Zn dạng bột sẽ làm tăng diện tích tiếp xúc, tốc độ phản ứng xảy ra nhanh hơn

Câu 1:
a) \(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(\dfrac{1}{2}Cl_2+K\underrightarrow{t^o}KCl\)
\(2KCl+2H_2O\xrightarrow[cómàngngăn]{đp}2KOH+Cl_2+H_2\)
\(Cl_2+H_2O⇌HCl+HClO\)
\(NaOH+HClO\rightarrow NaClO+H_2O\)
\(NaClO_{\left(rắn\right)}+HCl\rightarrow NaCl+Cl_2+H_2O\)
\(2NaCl+2H_2O\xrightarrow[cómàngngăn]{đp}2NaOH+Cl_2+H_2O\)
\(3Cl_2+2Fe\underrightarrow{t^o}2FeCl_3\)
b) \(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(Cl_2+H_2\underrightarrow{a/s}2HCl\)
\(2HCl+Fe\rightarrow FeCl_2+H_2\)
\(2FeCl_2+Cl_2\rightarrow2FeCl_3\)
\(FeCl_3+3KOH\rightarrow3KCl+Fe\left(OH\right)_3\downarrow\)
\(2KCl+2H_2O\xrightarrow[cmn]{đp}2KOH+Cl_2+H_2\)
\(6KOH+3Cl_2\underrightarrow{t^o}5KCl+KClO_3+3H_2O\)
\(KClO_3+6HCl\rightarrow KCl+3Cl_2+3H_2O\)
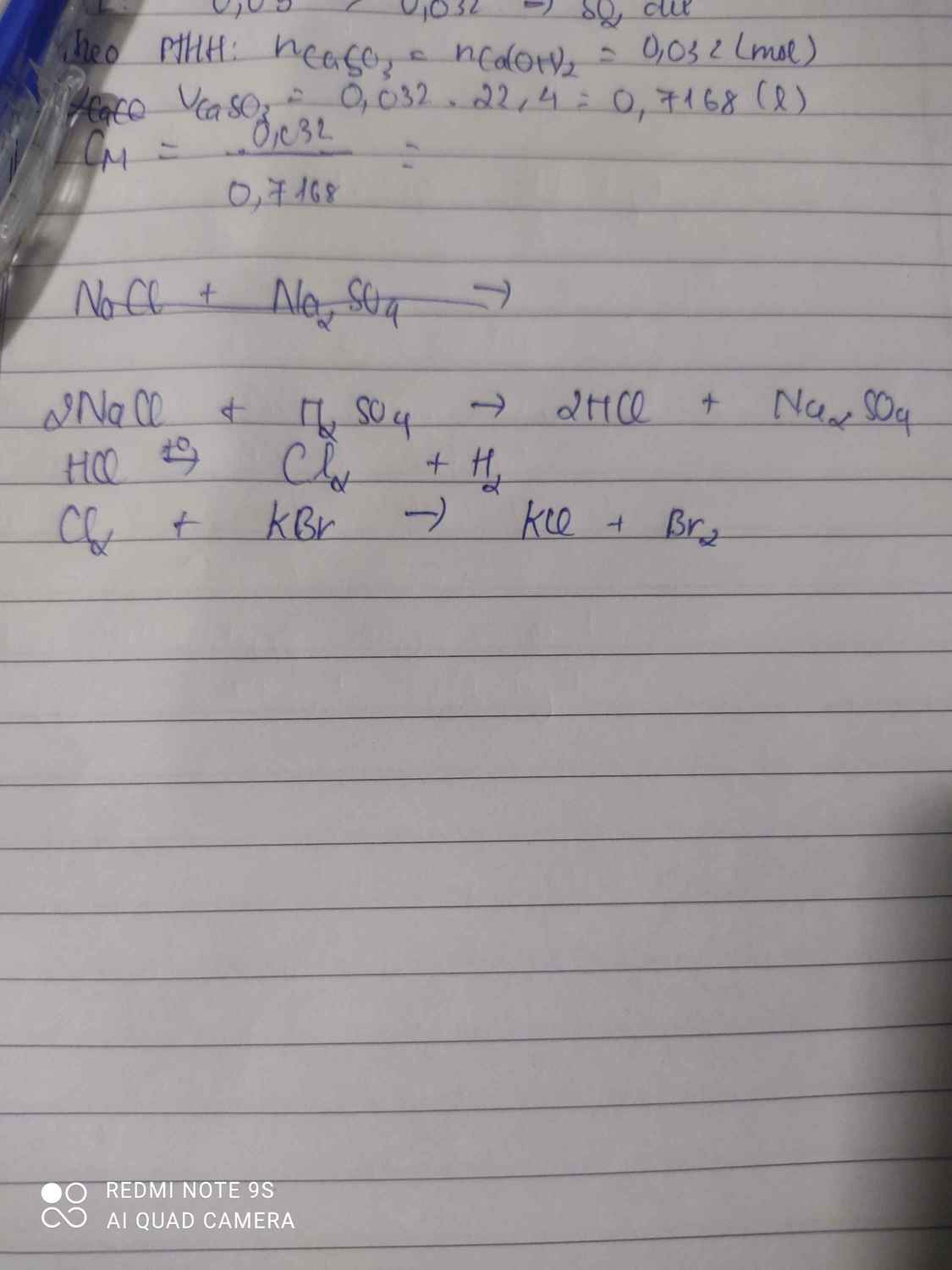

\(MnO_2+HCl\rightarrow Cl_2+MnCl_2+H_2O\)
\(Cl_2+2Na\rightarrow2NaCl\)
\(2NaCl+H_2SO_4\rightarrow2HCl+Na_2SO_4\)
từ NaCl chuyển thành HCl mà e