Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(CaCO_3-^{t^o}\rightarrow CaO+CO_2\)
\(n_{CaO}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
\(n_{CaCO_3}=n_{CaO}=0,1\left(mol\right)\\ \Rightarrow m_{CaCO_3}=0,1.100=10\left(g\right)\)
=> Chọn A

mCaCO3=80%.1,5=1,2(tấn)=1 200 000(gam)
nCaCO3= 1 200 000/100= 12000(mol)
PTHH: CaCO3 -to-> CaO + CO2
nCO2=nCaCO3=12000(mol)
=>V(CO2,dktc)=12000.22,4= 268800(l)

Muối cacbonat --phân huỷ--> CaO => muối đó là CaCO3
\(a,n_{CaO}=\dfrac{8,4}{56}=0,15\left(mol\right)\)
PTHH: \(CaCO_3\underrightarrow{t^o}CaO+CO_2\uparrow\)
0,15<----0,15
\(b,m_{CaCO_3}=0,15.100=15\left(g\right)\)

\(CuO+CO\rightarrow Cu+CO_2\)
..x..........x.........................
\(PbO+CO\rightarrow Pb+CO_2\)
..y........y........................
- Theo bài ra ta có hệ : \(\left\{{}\begin{matrix}80x+223y=3,83\\x+y=0,03\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}x=0,02\\y=0,01\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{CuO}=1,6\\m_{PbO}=2,23\end{matrix}\right.\) ( g )
b, \(n_K=n_{CO_2}=x+y=0,03\left(mol\right)\)
\(\Rightarrow V=0,672\left(l\right)\)
c, \(Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3+H_2O\)
........................0,03........0,03.............
\(\Rightarrow m_{kt}=3\left(g\right)\)
Đặt \(\left\{{}\begin{matrix}n_{CuO}=x\left(mol\right)\\n_{PbO}=y\left(mol\right)\end{matrix}\right.\)
\(m_{CuO}+m_{PbO}=3,83\\ \Rightarrow80x+223y=3,83\left(1\right)\)
\(PTHH:CuO+CO\underrightarrow{t^o}Cu+CO_2\uparrow\\ \left(mol\right)......x\rightarrow..x....x.....x\\ PTHH:PbO+CO\underrightarrow{t^o}Pb+CO_2\uparrow\\ \left(mol\right)......y\rightarrow..y....y.....y\\ n_{CO}=\dfrac{0,84}{28}=0,03\\ \Rightarrow x+y=0,03\left(2\right)\)
Từ (1) và (2) ta có hpt \(\left\{{}\begin{matrix}80x+223y=3,83\\x+y=0,03\end{matrix}\right.\)
Giải hpt ta được \(\left\{{}\begin{matrix}x=0,02\\y=0,01\end{matrix}\right.\)
\(a,\left\{{}\begin{matrix}m_{CuO}=80.0,02=1,6\left(g\right)\\m_{PbO}=3,83-1,6=2,23\left(g\right)\end{matrix}\right.\)
\(b,V_{CO_2}=\left(x+y\right).22,4=\left(0,02+0,01\right).22,4=0,672\left(l\right)\)
\(c,n_{CO_2}=x+y=0,02+0,01=0,03\left(mol\right)\\ PTHH:Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3\downarrow+H_2O\\ \left(mol\right)................0,03\rightarrow0,03\\ m_{CaCO_3}=0,03.100=3\left(g\right)\)

Ta có : nCu(OH)2=19,6/98=0,2 mol
PTHH:
Cu(OH)2 →to→ CuO + H2O
⇒nCu(OH)2=nCuO=0,2 mol
mCuO=0,2.80=16g
Đáp án:C

a, PT: \(MO+CO\underrightarrow{t^o}M+CO_2\)
Ta có: \(n_{MO}=\dfrac{7,2}{M_M+16}\left(mol\right)\)
\(n_M=\dfrac{5,6}{M_M}\left(mol\right)\)
Theo PT: \(n_{MO}=n_M\) \(\Rightarrow\dfrac{7,2}{M_M+16}=\dfrac{5,6}{M_M}\)
\(\Rightarrow M_M=56\left(g/mol\right)\)
⇒ M là Fe.
Vậy: Oxit kim loại đó là FeO.
b, Theo PT: \(n_{CO_2}=n_M=\dfrac{5,6}{56}=0,1\left(mol\right)\)
PT: \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_{3\downarrow}+H_2O\)
Theo PT: \(n_{CaCO_3}=n_{CO_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{\downarrow}=m_{CaCO_3}=0,1.100=10\left(g\right)\)
Bạn tham khảo nhé!
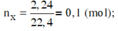
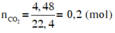
a) CaCO3--->CaO+CO2
n\(_{CaCO3}=\frac{60}{100}=0,6\left(mol\right)\)
Theo pthh
n\(_{CaO}=n_{CaC_{ }O3}=0,6\left(mol\right)\)
m\(_{CaO}=0,6.56=33,6\left(g\right)\)\
b)m\(_{C_{ }02}=0,6.44=26,4\left(g\right)\)
nCaCO3 = 0,6(mol)
PTHH : CaCO3--->CO2+CaO
=>nCaO = 0,6(mol)
=>mCaO = 0,6 . 56 = 33,6(g)
b,mCO2 = 0,6 . 44 = 26,4(g)