Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

CuO + 2HCl -> CuCl2 + H2O (1)
Fe2O3 + 6HCl -> 2FeCl3 + 3H2O (2)
nHCl=0,2.3,5=0,7(mol)
Đặt nCuO=a
nFe2O3=b
Ta có hệ:
80a+160b=20
2a+6b=0,7
=>a=0,05;b=0,1
mCuO=80.0,05=4(g)
mFe2O3=20-4=16(g)
Theo PTHH 1 và 2 ta có:
nCuCl2=nCuO=0,05(mol)
nFeCl3=2nFe2O3=0,2(mol)
mCuCl2=135.0,05=6,75(g)
mFeCl3=162,5.0,2=32,5(g)
mdd =20+200.1,1=240(g)
C% dd CuCl2=6,72\240 .100%=2,8125%
C% dd FeCl3= 32,5\240 .100%=13,54%

Câu 1:
PTHH: 2Al + 3H2SO4 ===> Al2(SO4)3 + 3H2
a)Vì Cu không phản ứng với H2SO4 loãng nên 6,72 lít khí là sản phẩm của Al tác dụng với H2SO4
=> nH2 = 6,72 / 22,4 = 0,2 (mol)
=> nAl = 0,2 (mol)
=> mAl = 0,2 x 27 = 5,4 gam
=> mCu = 10 - 5,4 = 4,6 gam
b) nH2SO4 = nH2 = 0,3 mol
=> mH2SO4 = 0,3 x 98 = 29,4 gam
=> Khối lượng dung dịch H2SO4 20% cần dùng là:
mdung dịch H2SO4 20% = \(\frac{29,4.100}{20}=147\left(gam\right)\)
nH2 = 6.72 : 22.4 = 0.3 mol
Cu không tác dụng với H2SO4
2Al + 3H2SO4 -> Al2(SO4)3 + 3H2
0.2 <- 0.3 <- 0.1 <- 0.3 ( mol )
mAl = 0.2 x 56 = 5.4 (g)
mCu = 10 - 5.4 = 4.6 (g )
mH2SO4 = 0.3 x 98 = 29.4 ( g)
mH2SO4 20% = ( 29.4 x100 ) : 20 = 147 (g)

Mg + 2HCl \(\rightarrow\) MgCl2 + H2
x.........2x............x............x
MgO + 2HCl \(\rightarrow\) MgCl2 + H2O
y............2y............y
a, m hỗn hợp = 8,8 (g)
\(\Rightarrow\) 24x + 40y = 8,8 (1)
n MgCl2 = 28,5 : 95 = 0,3 mol
\(\Rightarrow\) x + y = 0,3 (2)
GIải hệ (1),(2) : x = 0,2 ; y = 0,1
m Mg = 24 . 0,2 = 4,8 g
% m Mg = 4,8 : 8,8 . 100% = 54,5%
% m MgO = 100% - 54,5% = 45,5%
b, n HCl = 2x + 2y = 2.0,2 + 2.0,1= 0,6 mol
m HCl = 0,6 . 36,5 = 21,9 g
m dd HCl = 21,9 . 100 : 14,6 = 150 g
c, n H2 = x = 0,2 mol
m H2 = 0,2 . 2 = 0,4 g
m dd thu được sau phản ứng
= m hỗn hợp + m dd HCl - m H2
= 8,8 + 150 - 0,4
= 158,4 g
C%MgCl2 = 28,5 : 158,4 . 100% = 18%

\(n_{CO_2}=\dfrac{0,448}{22,4}=0,02(mol)\\ a,CaCO_3+2HCl\to CaCl_2+H_2O+CO_2\uparrow\\ b,n_{CaCO_3}=n_{CO_2}=0,02(mol)\\ \Rightarrow m_{CaCO_3}=0,02.100=2(g)\\ c,\%_{CaCO_3}=\dfrac{2}{5}.100\%=40\%\\ \%_{CaSO_4}=100\%-40\%=60\%\)

PT: \(Mg+H_2SO_4\rightarrow MgSO_4+H_2\)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
a, Giả sử: \(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\n_{Al}=y\left(mol\right)\end{matrix}\right.\)
⇒ 24x + 27y = 12,6 (1)
Ta có: \(n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Mg}+\dfrac{3}{2}n_{Al}=x+\dfrac{3}{2}y\left(mol\right)\)
\(\Rightarrow x+\dfrac{3}{2}y=0,6\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,3\left(mol\right)\\y=0,2\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{MG}=\dfrac{0,3.24}{12,6}.100\%\approx57,1\%\\\%m_{Al}\approx42,9\%\end{matrix}\right.\)
b, Theo PT: \(\left\{{}\begin{matrix}n_{H_2SO_4}=n_{H_2}=0,6\left(mol\right)\\n_{MgSO_4}=n_{Mg}=0,3\left(mol\right)\\n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{H_2SO_4}=0,6.98=58,8\left(g\right)\Rightarrow m_{ddH_2SO_4}=\dfrac{58,8}{14,7\%}=400\left(g\right)\)
Ta có: m dd sau pư = 12,6 + 400 - 0,6.2 = 411,4 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{MgSO_4}=\dfrac{0,3.120}{411,4}.100\%\approx8,75\%\\C\%_{Al_2\left(SO_4\right)_3}=\dfrac{0,1.342}{411,4}.100\%\approx8,31\%\end{matrix}\right.\)
Bạn tham khảo nhé!

\(n_{H_2}=\dfrac{6,1975}{24,79}=0,25(mol)\\ a,PTHH:Mg+2HCl\to MgCl_2+H_2\\ MgO+2HCl\to MgCl_2+H_2\\ b,n_{Mg}=n_{H_2}=0,25(mol)\\ \Rightarrow \%_{Mg}=\dfrac{0,25.24}{12}.100\%=50\%\\ \%_{MgO}=100\%-50\%=50\%\\ c,n_{MgO}=\dfrac{12-0,25.24}{40}=0,15(mol)\\ \Rightarrow \Sigma n_{HCl}=0,25.2+0,15.2=0,8(mol)\\ \Rightarrow x=C_{M_{HCl}}=\dfrac{0,8}{0,4}=2M\)
\(d,n_{MgCl_2}=0,25+0,15=0,4(mol)\\ \Rightarrow m_{MgCl_2}=0,4.95=38(g)\)

nH2=0,1(mol)
PTHH: Mg + 2 HCl -> MgCl2 + H2
0,1__________0,2___________0,1(mol)
MgO + 2 HCl -> MgCl2 + H2O
0,05____0,1___0,05(mol)
mMg=0,1. 24= 2,4(g) -> mMgO=4,4-2,4= 2(g) -> nMgO=0,05((mol)
b) %mMg= (2,4/4,4).100=54,545%
=> %mMgO=45,455%
c) nHCl=0,3(mol) -> mHCl=0,3.36,5=10,95(g)
=> mddHCl=(10,95.100)/7,3=150(g)

\(a)n_{HCl}=0,2.1,5=0,3mol\\ CaO+2HCl\rightarrow CaCl_2+H_2O\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ \Rightarrow\left\{{}\begin{matrix}2n_{CaO}+2n_{CuO}=0,3\\56n_{CaO}+80n_{CuO}=10,8\end{matrix}\right.\\ \Rightarrow n_{CaO}=n_{CaCl_2}=0,05mol;n_{CuO}=n_{CuCl_2}=0,1mol\\ \%m_{CaO}=\dfrac{0,05.56}{10,8}\cdot100=25,93\%\\ \%m_{CuO}=100-25,93=74,07\%\\ b)C_{M_{CaCl_2}}=\dfrac{0,05}{0,2}=0,25M\\ C_{M_{CuCl_2}}=\dfrac{0,1}{0,2}=0,5M\)
\(CaO+2HCl\rightarrow CaCl_2+H_2O\)
x 2x x x
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
y 2y y y
\(\left\{{}\begin{matrix}56x+80y=10,8\\2x+2y=0,2.1,5=0,3\end{matrix}\right.\)
\(\Rightarrow x=0,05;y=0,1\)
\(a,\%m_{CaO}=0,05.56:10,8.100\%=25,93\left(\%\right)\)
\(\%m_{CuO}=100\%-25,93\%=74,07\%\)
\(b,C_{M\left(CaCl_2\right)}=\dfrac{0,05}{0,2}=0,25\left(M\right)\)
\(C_{M\left(CuCl_2\right)}=\dfrac{0,1}{0,2}=0,5\left(M\right)\)

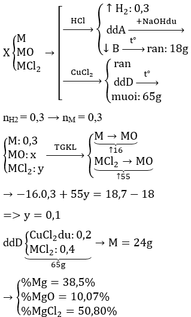
a) CaO + 2HCl --> CaCl2 + H2O
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
b) \(n_{CO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
_____0,1<-----0,2<-------0,1<-----0,1
=> mCaCO3 = 0,1.100 = 10 (g)
=> mCaO = 15,6 - 10 = 5,6 (g)
b) \(n_{CaO}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
PTHH:CaO + 2HCl --> CaCl2 + H2O
_____0,1--->0,2------>0,1
=> mHCl = (0,2+0,2).36,5 = 14,6 (g)
=> \(m_{ddHCl}=\dfrac{14,6.100}{14,6}=100\left(g\right)\)
mdd sau pư = 15,6 + 100 - 0,1.44 = 111,2 (g)
=> \(C\%\left(CaCl_2\right)=\dfrac{\left(0,1+0,1\right).111}{111,2}.100\%=19,96\%\)
PTHH : CaO + 2HCl ---> CaCl2 + H2O (1)
1 : 2 : 1 : 2
CaCO3 + 2HCl ---> CaCl2 + H2O + CO2 (2)
1 : 2 : 1 : 1 : 1
Ta có \(n_{CO_2}=\dfrac{V}{22.4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
=> \(n_{CaCO_3}=0,1\left(mol\right)\)
=> \(m_{CaCO_3}=n.M=0,1.100=10\left(g\right)\)
=> mCaO = 15,6 - 10 = 5,6 (g)
c) \(n_{CaO}=\dfrac{m}{M}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
\(m_{CO_2}=n.M=0,1.44=4,4\left(g\right)\)
Ta có \(m_{HCl}=m_{HCl\left(1\right)}+m_{HCl\left(2\right)}\)
\(=n_{HCl\left(1\right)}.M+n_{HCl\left(2\right)}.M\)
\(0,2.36,5+0,2.36,5=14,6\left(g\right)\)
=> \(m_{ddHCl}=\dfrac{m_{HCl}.100\%}{C\%}=\dfrac{14,6.100\%}{14.6\%}100\left(g\right)\)
\(m_{dd\text{ sau pư}}=m_{ddHCl}+m_{CaO}+m_{CaCO_3}-m_{CO_2}\)
= 100 + 5.6 + 10 - 4,4 = 111.2(g)
=> \(m_{CaCl_2}=m_{CaCl_2\left(1\right)}+m_{CaCl_2\left(2\right)}\)
\(=n_{CaCl_2\left(1\right)}.M+n_{CaCl_2\left(2\right)}.M\)
= 0,1.91 + 0,1.91 = 18,2 (g)
=> \(C\%=\dfrac{m_{CaCl_2}}{m_{\text{dd sau pư}}}.100\%=\dfrac{18,2}{111,2}.100\%=16,37\%\)