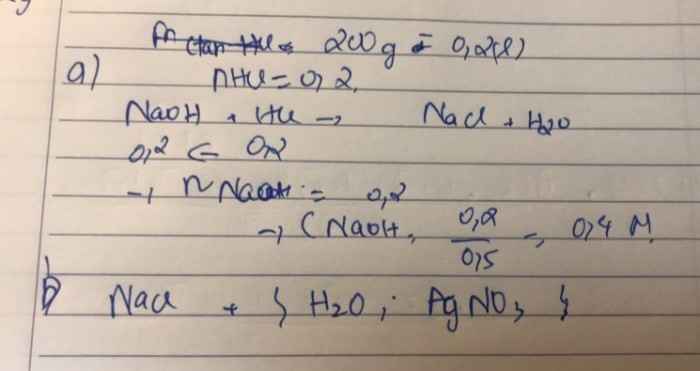Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{Na}=\dfrac{13,8}{23}=0,6\left(mol\right)\\ pthh:Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
0,6 0,6 0,3
\(V_{H_2}=0,3.22,4=6,72\left(l\right)\\ c,m_{\text{dd}}=13,8+286,8-\left(0,3.2\right)=300\left(g\right)\\ C\%=\dfrac{0,6.40}{300}.100\%=8\%\)
\(n_{Na}\) = \(\dfrac{13,8}{23}\) = 0,6 mol
Theo PTHH:
a) \(2Na+2H_2O\underrightarrow{t^o}2NaOH+H_2\)
2 2 2 1 (mol)
0,6 \(\rightarrow\) 0,6 \(\rightarrow\) 0,6 \(\rightarrow\) 0,3 (mol)
b) \(V_{H_2}\) = 0,3.22,4 = 6,72l
c) \(m_{dd}\) = 13,8 + 286,8 - 0,3.2 = 300g
\(C\%\) = \(\dfrac{0,6.40}{300}\).100% = 8%

Đặt nMg=a(mol); nAl=b(mol)
PTHH: Mg +2 HCl -> MgCl2 + H2
a________2a_______a_____a(mol)
2 Al + 6 HCl -> 2 AlCl3 +3 H2
b_____3b____b_____1,5b(mol)
Ta có hpt: \(\left\{{}\begin{matrix}24a+27b=7,8\\a+1,5b=0,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,2\end{matrix}\right.\)
=> %mMg=[(0,1.24)/7,8].100=30,769%
=>%mAl= 69,231%
c) MgCl2 + 2 NaOH -> Mg(OH)2 + 2 NaCl
0,1_______________0,1(mol)
AlCl3 + 3 NaOH -> Al(OH)3 + 3 NaCl
0,2____________0,2(mol)
=> m=m(kết tủa)= mMg(OH)2+ mAl(OH)3= 58.0,1+ 78.0,2= 21,4(g)

\(n_{NaOH}=2.0,5=1\left(mol\right)\)
\(n_{KOH}=1.0,5=0,5\left(mol\right)\)
=> Chất tan có trong dung dịch thu được: \(\left\{{}\begin{matrix}NaOH:1\left(mol\right)\\KOH:0,5\left(mol\right)\end{matrix}\right.\)
\(m_{ddNaOH}=1,2.500=600\left(g\right)\)
\(m_{ddKOH}=1,2.500=600\left(g\right)\)
\(\Rightarrow m_{ddsau}=600+600=1200\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{NaOH}=\dfrac{1.40.100}{12000}=\dfrac{1}{3}\%\\C\%_{KOH}=\dfrac{0,5.56.100}{12000}=\dfrac{7}{30}\%\end{matrix}\right.\)
\(V_{ddsau}=0,5+0,5=1\left(l\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C_{M_{NaOH}}=\dfrac{1}{1}=1\left(M\right)\\C_{M_{KOH}}=\dfrac{0,5}{1}=0,5\left(M\right)\end{matrix}\right.\)
mk làm mà ko chắc đâu nhé,mong mọi người góp ý
áp dụng sơ đồ đường chéo
0,5l dd NaOH 2M------------x-1 ( M)
--------------------------x(M)
0,5l dd KOH 1 M--------------2-x (M)
Ta có x-1= 2-x => x= 1,5 M
mdd NaOH = 500.1,2=600 gam
mdd KOH =500.1,2=600 gam
mNaOH= (0,5.2).40=40 gam ->C%(NaOH)= 40.100%/600=20/3%
mKOH=(0,5.1).56=28 gam -> C%(KOH) =28.100%/600=14/3 %
Áp dụng sơ đồ đường chéo ta tính được
C%=5,666%

Sửa đề 200ml dd HCl
\(a,n_{HCl}=0,2.0,1=0,02\left(mol\right)\)
PTHH: \(NaOH+HCl\rightarrow NaCl+H_2O\)
0,02<----0,02
\(C_{M\left(NaOH\right)}=\dfrac{0,02}{0,5}=0,04M\)
b) Muối thu được là NaCl phản ứng được với H2O, AgNO3
\(2NaCl+2H_2O\xrightarrow[\text{có màng ngăn}]{\text{điện phân}}2NaOH+Cl_2\uparrow+H_2\uparrow\\ NaCl+AgNO_3\rightarrow AgCl\downarrow+NaNO_3\)

\(n_{Na}=0.02\left(mol\right)\)
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
\(0.02....................0.02........0.01\)
\(V_{H_2}=0.01\cdot22.4=0.224\left(l\right)\)
\(m_{NaOH}=0.02\cdot40=0.8\left(g\right)\)
\(C\%_{NaOH}=\dfrac{0.8}{0.46+200-0.01\cdot2}\cdot100\%=0.4\%\)

\(m_{H_2O}=37,6.1=37,6\left(g\right)\\ m_{ddNaOH}=12,4+37,6=50\left(g\right)\\ C\%_{ddNaOH}=\dfrac{12,4}{50}.100=24,8\%\)
Ta có: \(D_{H_2O}=1\left(g/mol\right)\Rightarrow m_{H_2O}=37,6.1=37,6\left(g\right)\)
\(\Rightarrow C\%_{ddNaOH}=\dfrac{12,4.100\%}{37,6}=32,98\%\)

a, Theo de bai ta co
mct=mNaCl=15g
mdm=mH2O=65g
mdd=mct+mdm=15+65=80g
\(\Rightarrow\) Nong do % dd thu duoc la
C%=\(\dfrac{mct}{mdd}.100\%=\dfrac{15}{80}.100\%=18,75\%\)
b, Theo de bai ta co
So gam NaCl can dung de pha che la
mNaCl=\(\dfrac{mdd.C\%}{100}\dfrac{120.12}{100}=14,4g\)
So gam nuoc can dung la
mH2O=mdm=mdd-mct=120-14,4=105,6 g
c, Theo de bai ta co
mdd=mct+mH2O=6+144=150g
Nong do % cua dd NaOH thu duoc la
C%=\(\dfrac{mct}{mdd}.100\%\)\(=\dfrac{6}{150}.100\%=4\%\)
Theo de bai ta co
So mol cua NaOH
nNaOH=\(\dfrac{6}{40}=0,15mol\)
The tich cua dd NaOH la
V=\(\dfrac{m}{D}=\dfrac{150}{1,2}=125ml=0,125l\)
\(\Rightarrow\)Nong do mol cua dd la
CM=\(\dfrac{n}{V}=\dfrac{0,15}{0,125}=1,2M\)
\(a)\)
\(C\%NaOH=\dfrac{15}{15+65}.100\%=18,75\%\)
\(b)\)
Ta có: \(12\%=\dfrac{m_{NaOH}}{120}.100\%\)
\(\Rightarrow m_{NaOH}=14,4\left(g\right)\)
\(c)\)
\(C\%NaOH=\dfrac{6}{6+144}.100\%=4\%\)
\(C_{M_{NaOH}}=\dfrac{10.C\%_{NaOH}.D_{NaOH}}{M_{NaOH}}=\dfrac{10.4.1,2}{40}=1,2\left(M\right)\)