Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Zn+2HCl→ZnCl\(_2\)+H\(_2\)
0,1 0,2 0,1(mol)
ZnO+2HCl→ZnCl\(_2\)+H\(_2\)O
0,05 0,1 (mol)
n\(H_2\)=\(\dfrac{2,24}{22,4}\)=0,1(mol)
m\(_{Zn}\)=0,1.65=6,5(g)
m\(_{ZnO}\)=10,55-6,5=4,05(g)
b/
n\(_{HCl}\)=0,1+0,2=0,3(mol)
m\(_{HCl}\)=0,3.36,5=10,95(g)
m\(_{dd_{HCl}}\)=\(\dfrac{100.10,95}{10}\)=109,5(g)
n\(_{ZnO}\)=\(\dfrac{4,05}{81}\)=0,05(mol)
cho mình hỏi tại sao HCl ở pt dưới ra được 0,1 mol vậy..

nH2 = 6,72/22,4 = 0,3 (mol)
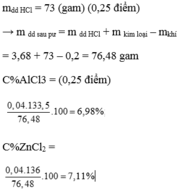
PTHH: 2Al + 6HCl -> 2AlCl3 + 3H2
nAl = 0,3 : 3 . 2 = 0,2 (mol)
nHCl (Al) = 0,3 . 2 = 0,6 (mol)
mAl = 0,2 . 27 = 5,4 (g)
%mAl = 5,4/25,65 = 20,05%
%mZnO = 100% - 20,05% = 79,95%
mZnO = 25,65 - 5,4 = 20,25 (g)
nZnO = 20,25/81 = 0,25 (mol)
PTHH: ZnO + 2HCl -> ZnCl2 + H2O
nHCl (ZnO) = 0,25 . 2 = 0,5 (mol)
nHCl (đã dùng) = 0,6 + 0,5 = 1,1 (mol)
CMddHCl = 1,1/0,1008 = 10,9M
C% = (10,9 . 36,5)/(10 . 1,19) = 33,43%

a.\(n_{H_2}=\dfrac{7,28}{22,4}=0,325mol\)
Gọi \(\left\{{}\begin{matrix}n_{Al}=x\\n_{Zn}=y\end{matrix}\right.\) \(\left(mol\right)\) \(\rightarrow27x+65y=10,55\left(g\right)\) (1)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
x 1/2 x 3/2 x ( mol )
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
y y y ( mol )
\(\rightarrow\dfrac{3}{2}x+y=0,325\left(mol\right)\) (2)
\(\left(1\right);\left(2\right)\rightarrow\left\{{}\begin{matrix}x=0,15\\y=0,1\end{matrix}\right.\)
\(\rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,15.27}{10,55}.100\%=38,38\%\\\%m_{Zn}=100\%-38,38\%=61,62\%\end{matrix}\right.\)
b.\(\left\{{}\begin{matrix}n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}.0,15=0,075\\n_{ZnSO_4}=0,1\end{matrix}\right.\) ( mol )
\(\left\{{}\begin{matrix}C_{M_{Al_2\left(SO_4\right)_3}}=\dfrac{0,075}{0,8}=0,09M\\C_{M_{ZnSO_4}}=\dfrac{0,1}{0,8}=0,125M\end{matrix}\right.\)

a) \(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: Zn + H2SO4 --> ZnSO4 + H2
0,2<----------------0,2<---0,2
\(\left\{{}\begin{matrix}\%m_{Zn}=\dfrac{0,2.65}{16,2}.100\%=80,247\%\\\%m_{Cu}=100\%-80,247\%=19,753\%\end{matrix}\right.\)
b) \(m_{ZnSO_4}=0,2.161=32,2\left(g\right)\)