Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{PbS}=\dfrac{47,8}{239}=0,2\left(mol\right)\)
Bảo toàn S: \(n_{FeS}=n_{H_2S}=0,2\left(mol\right)\)
\(n_{H_2}=\dfrac{6,72}{22,4}-0,2=0,1\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,1<----------------------0,1
=> mFe = 0,1.56 = 5,6 (g)
mFeS = 0,2.88 = 17,6 (g)
\(\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{5,6}{5,6+17,6}.100\%=24,138\%\\\%m_{FeS}=\dfrac{17,6}{5,6+17,6}.100\%=75,862\%\end{matrix}\right.\)

\(n_{HCl}=\frac{60.36,5\%}{36,5}=0,6\left(mol\right)\)
\(FeS+2HCl\rightarrow FeCl_2+H_2S\)
\(Na_2S+2HCl\rightarrow2NaCl+H_2S\)
Gọi a là mol FeS; b là mol Na2S
\(\left\{{}\begin{matrix}88a+78b=25,4\\2a+2b=0,6\rightarrow\end{matrix}\right.\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\)
\(\%_{FeS}=\frac{0,2.88.100}{25,4}=69,29\%\)
\(\%_{Na2S}=100\%-69,29\%=30,71\%\)
\(n_{FeCl2}=0,2\left(mol\right);n_{NaCl}=0,2\left(mol\right)\)
\(\rightarrow n_{Cl}=2n_{FeCl2}+n_{NaCl}=0,6\left(mol\right)\)
\(PTHH:Pb+2Cl\rightarrow PbCl_2\)
\(\rightarrow n_{PbCl2}=0,3\left(mol\right)\)
\(\rightarrow m+PbCl2=83,4\left(g\right)\)

mình cần giải gấp khoảng 1 tiếng ai có thể giải giùm mình được ko

\(Đặt:n_{MnO_2}=a\left(mol\right),n_{KMnO_4}=b\left(mol\right)\)
\(m_{hh}=87a+158b=37.96\left(g\right)\left(1\right)\)
\(n_{Cl_2}=\dfrac{10.08}{22.4}=0.45\left(mol\right)\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(n_{Cl_2}=a+2.5b=0.45\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.4,b=0.02\)
\(\%MnO_2=\dfrac{0.4\cdot87}{37.96}\cdot100\%=91.68\%\\\%KMnO_4=100-91.68=8.32\% \)
\(m_M=m_{KCl}+m_{MnCl_2}=0.02\cdot74.5+\left(0.4+0.02\right)\cdot126=54.41g\)

Đáp án B
nSO2 = 1,7 (mol)
Chất rắn Z là Fe2O3, nFe2O3 = 0,4 (mol)
2Febđ → Fe2O3
0,8 ← 0,4 (mol)
Ta có: mX = 1,7 ×64 – 48=60,8 (gam)
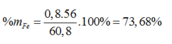



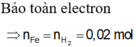
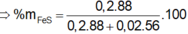

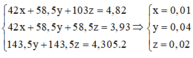
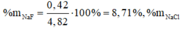
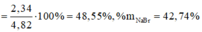
Đặt \(n_{FeS}=a\left(mol\right);n_{Na_2S}=b\left(mol\right)\)
\(\left(1\right)FeS+2HCl\rightarrow FeCl_2+H_2S\)
(mol)_a______2a_____a________a_
\(\left(2\right)Na_2S+2HCl\rightarrow2NaCl+H_2S\)
(mol)__b_____2b_______2b_____b__
Theo đề ta có hpt:
\(\left\{{}\begin{matrix}88a+78b=25,4\\36,5\left(2a+2b\right)=\frac{60.36,5}{100}\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}\%m_{FeS}=\frac{0,2.88}{25,4}.100\%=69,3\left(\%\right)\\\%m_{Na_2S}=100-69,3=30,7\left(\%\right)\end{matrix}\right.\)
\(PTHH:FeCl_2+Pb\left(NO_3\right)_2\rightarrow PbCl_2+Fe\left(NO_3\right)_2\)
(mol)_____0,2___________________0,2______________
\(PTHH:2NaCl+Pb\left(NO_3\right)_2\rightarrow PbCl_2+2NaNO_3\)
(mol)______0,2__________________0,1____________
\(m_{\downarrow}=\left(0,2+0,1\right).278=83,4\left(g\right)\)
\(\text{Đặt }n_{FeS}=a\left(mol\right);n_{Na_2S}=b\left(mol\right)\\ \Rightarrow m_{h^2}=88a+78b=25,4\left(1\right)\\ BTNT.S\Rightarrow n_{S^{2-}}=n_{Na_2S}+n_{FeS}=a+b\left(mol\right)\\ n_{HCl}=0,6\left(mol\right)\)
Vai trò H+ : 2H+ + S2- ---> H2S
__________0,6___0,3
\(\Rightarrow n_{S^{2-}}=a+b=0,3\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\)
\(\Rightarrow m_{FeS}=17,6\left(g\right);m_{Na_2S}=7,8\left(g\right)\\ \Rightarrow\%FeS=69,29\%;\%Na_2S=30,71\%\)
b) BTNT.Cl \(\Rightarrow n_{PbCl_2}=\frac{1}{2}n_{HCl}=0,3\left(mol\right)\)
\(\Rightarrow m_{PbCl_2}=83,4\left(g\right)\)