
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_A=\dfrac{16,8}{M_A}\left(mol\right)\)
PTHH: 2xA + yO2 --to--> 2AxOy
\(\dfrac{16,8}{M_A}\)------------>\(\dfrac{16,8}{x.M_A}\)
=> \(\dfrac{16,8}{x.M_A}=\dfrac{23,2}{x.M_A+16y}\)
=> \(M_A=21.\dfrac{2y}{x}\)
Xét \(\dfrac{2y}{x}=1=>L\)
Xét \(\dfrac{2y}{x}=2=>L\)
Xét \(\dfrac{2y}{x}=3=>L\)
Xét \(\dfrac{2y}{x}=\dfrac{8}{3}\Rightarrow M_A=56\left(Fe\right)\)

\(nCu=\dfrac{3,2}{64}=0,05mol\)
pthh \(2Cu+O_2\underrightarrow{t^o}2CuO\)
=>\(nO_2=\dfrac{1}{2}.0,05=0,025mol\)
\(VO_2=0,025.22,4=0,56lít\)
\(Vkk=0,56:\dfrac{1}{5}=2,8lít\)
=> \(nCuO=nCu=0,05mol\)
\(mCuO=0,05.80=4gam\)

- Số mol Al là: nAl=m.M=13,5.27=0,5(mol)
PTHH:4Al+3O2→2Al2O3
(mol) 4 3 2
(mol) 0,5 0,375 0,25
Thể tích của khí Oxi cần dùng là:
VO2=n.22,4=0,375.22,4=8,4(l)
\(n_{Al}=\dfrac{13,5}{27}=0,5\left(mol\right)\\ n_{Al_2O_3}=\dfrac{20,4}{102}=0,2\left(mol\right)\\ 4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ Vì:\dfrac{0,5}{4}>\dfrac{0,2}{2}\Rightarrow Aldư\\ \Rightarrow n_{O_2}=\dfrac{3}{2}.n_{Al_2O_3}=\dfrac{3.0,2}{2}=0,3\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\)

PTHH : \(4Al+3O_2\left(t^o\right)->2Al_2O_3\) (1)
\(n_{Al_2O_3}=\dfrac{m}{M}=\dfrac{20,4}{27.2+16.3}=0,2\left(mol\right)\)
Từ (1) -> \(n_{O_2}=\dfrac{3}{2}n_{Al_2O_3}=0,3\left(mol\right)\)
-> \(V_{O_2\left(đktc\right)}=n.22,4=0,3.22,4=6,72\left(l\right)\)

PT: \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
_____0,9___0,6______0,3 (mol)
a, \(V_{O_2}=0,6.22,4=13,44\left(l\right)\)
b, \(m_{Fe}=0,9.56=50,4\left(g\right)\)
c, \(V_{kk}=\dfrac{V_{O_2}}{20\%}=67,2\left(l\right)\)

a, \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
b, Ta có: \(n_{Fe}=\dfrac{50,4}{56}=0,9\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{2}{3}n_{Fe}=0,6\left(mol\right)\Rightarrow V_{O_2}=0,6.22,4=13,44\left(l\right)\)
c, \(n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=0,3\left(mol\right)\Rightarrow m_{Fe_3O_4}=0,3.232=69,6\left(g\right)\)
d, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,4\left(mol\right)\Rightarrow m_{KClO_3}=0,4.122,5=49\left(g\right)\)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{50,4}{56}=0,9\left(mol\right)\)
\(a.PTHH:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
3 2 1
0,9 0,6 0,3
\(b.V_{O_2}=n.24,79=0,6.24,79=14,874\left(l\right)\)
\(c.m_{Fe_3O_4}=n.M=0,3.\left(56.3+16.4\right)=69,6\left(g\right)\)
\(d.V_{O_2}=14,874\left(l\right)\\ \Rightarrow n_{O_2}=\dfrac{V}{24,79}=\dfrac{14,874}{24,79}=0,6\left(mol\right)\\ PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
2 2 3
0,6 0,6 0,9
\(m_{KClO_3}=n.M=0,6.\left(39+35,5+16.3\right)=55,5\left(g\right).\)

Ta có: \(n_{P_2O_5}=\dfrac{14,2}{142}=0,1\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
___0,2_________0,1 (mol)
\(\Rightarrow m_{P\left(pư\right)}=0,2.31=6,2\left(g\right)\)
Mà: mP (ban đầu) = 6,89 (g)
\(\Rightarrow H\%=\dfrac{6,2}{6,89}.100\%\approx89,99\%\)
Bạn tham khảo nhé!

nFe = 2.8/56 = 0.05 (mol)
nO2 = 22.4 / 22.4 = 1 (mol)
3Fe + 2O2 -to-> Fe3O4
0.05__1/30______1/60
mO2 (dư) = ( 1 - 1/30) * 32 = 30.93 (g)
mFe3O4 = 1/60 * 232 = 3.867 (g)
a/ \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
b/ Ta có: \(n_{Fe}=\dfrac{2.8}{56}=0.05\left(mol\right)\)
\(n_{O_2}=\dfrac{22.4}{22.4}=1\left(mol\right)\)
Ta có: \(\dfrac{n_{Fe\left(bra\right)}}{n_{Fe\left(pt\right)}}=\dfrac{0.05}{3}=0.016< \dfrac{n_{O_2\left(bra\right)}}{n_{O_2\left(pt\right)}}=\dfrac{1}{2}=0.5\)
=> Oxi phản ứng dư
mO2 dư = (1 - 1/30) . 32 = 30.93 (g)
mFe3O4 = 1/60 . 232 = 3.867 (g)

PTHH: \(3Fe+2O_2\xrightarrow[]{t^o}Fe_3O_4\)
Ta có: \(n_{Fe_3O_4}=\dfrac{34,8}{232}=0,15\left(mol\right)\)
\(\Rightarrow n_{Fe}=0,45\left(mol\right)\) \(\Rightarrow m_{Fe}=0,45\cdot56=25,2\left(g\right)\)
\(3Fe+2O_2\rightarrow Fe_3O_4\)
0,15 mol
\(n_{Fe_3O_4}=\dfrac{m_{Fe_3O_4}}{M_{Fe_3O_4}}=\dfrac{34,8}{232}=0,15\left(mol\right)\)
\(\Rightarrow n_{Fe}=3n_{Fe_3O_4}=0,45\left(mol\right)\)
\(\Rightarrow m_{Fe}=n_{Fe}.M_{Fe}=0,45.56=25,2\left(g\right)\)
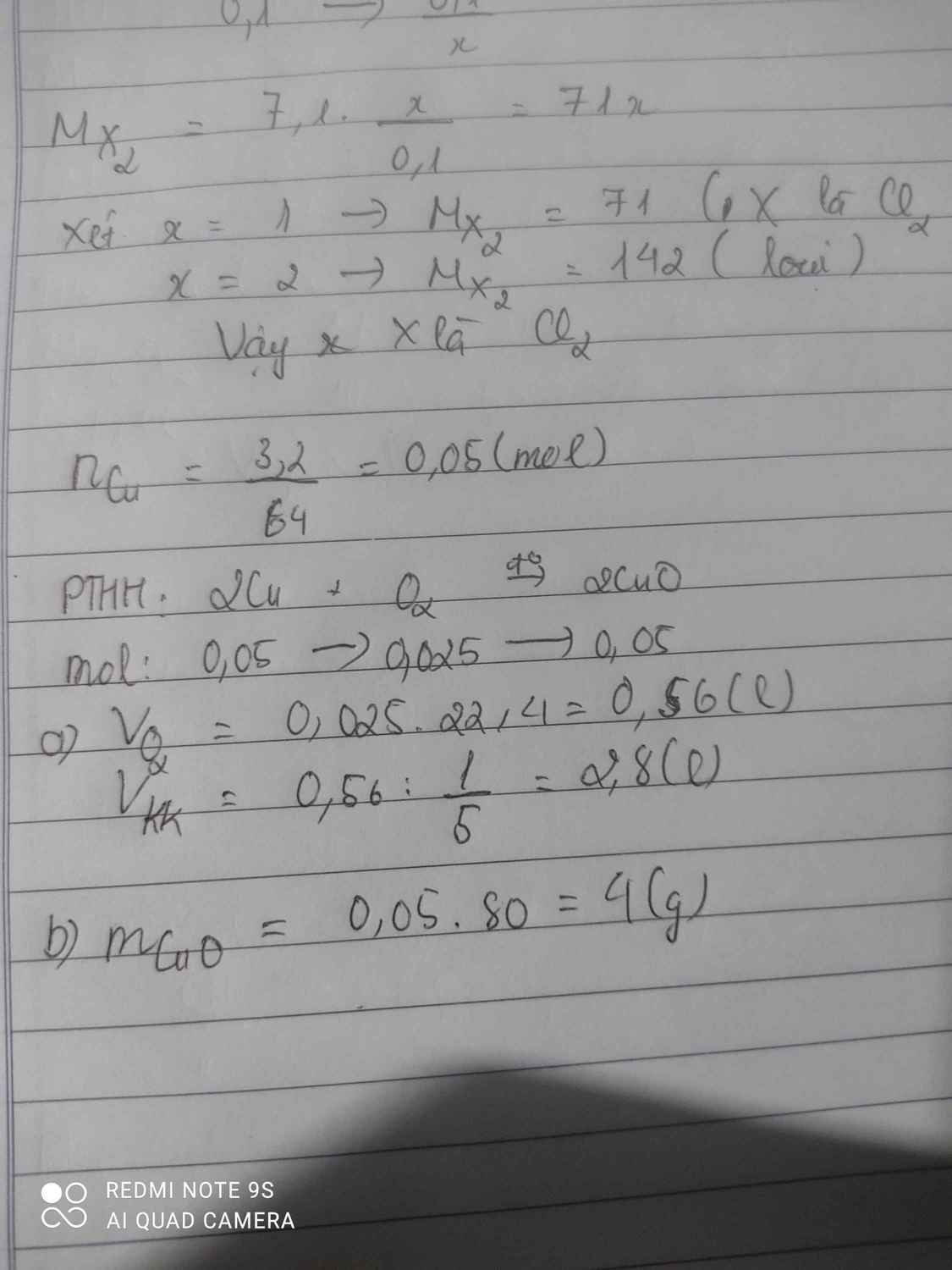
TK
PTHH:3Fe+2O2----->Fe3O4
a.nFe3O4=mFe3O4MFe3O4=46,4232=0,2(mol)nFe3O4=mFe3O4MFe3O4=46,4232=0,2(mol)
Theo PTHH:nO2=2nFe3O4=2.0,2=0,4(mol)nO2=2nFe3O4=2.0,2=0,4(mol)
VO2=nO2.22,4=0,4.22,4=8,96(l)VO2=nO2.22,4=0,4.22,4=8,96(l)
b.Theo PTHH:nFe=3nFe3O4=3.0,2=0,6(mol)nFe=3nFe3O4=3.0,2=0,6(mol)
mFe=nFe.MFe=0,6.56=33,6(g)mFe=nFe.MFe=0,6.56=33,6(g)
c.PTHH:4Al+3O2----->2Al2O3
Theo PTHH:nAl=43nO2=43.0,4=815(mol)nAl=43nO2=43.0,4=815(mol)
mAl=nAl.MAl=815.27=14,4(g)
Đề hỏi gì em ơi?