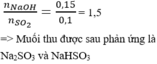Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{AlCl_3}=\dfrac{6,675}{133,5}=0,05\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,05<-----------0,05---->0,075
=> \(\%Al=\dfrac{0,05.27}{14,15}.100\%=9,54\%\)
=> \(\%Cu=\dfrac{14,15-0,05.27}{14,15}.100\%=90,46\%\)
b) \(V_{H_2}=0,075.22,4=1,68\left(l\right)\)
c) \(n_{Cu}=\dfrac{14,15-0,05.27}{64}=0,2\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,05->0,0375
2Cu + O2 --to--> 2CuO
0,2-->0,1
=> \(V_{O_2}=\left(0,1+0,0375\right).22,4=3,08\left(l\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\\ m_{AlCl_3}=6,675\left(mol\right)\\ n_{AlCl_3}=\dfrac{6,675}{133,5}=0,05\left(mol\right)\\ \Rightarrow n_{Al}=n_{AlCl_3}=0,05\left(mol\right)\\ \Rightarrow m_A=0,05.27=1,35\left(g\right);m_{Cu}=14,15-1,35=12,8\left(g\right)\\ \%m_{Cu}=\dfrac{12,8}{14,15}.100\approx90,459\%\\ \Rightarrow\%m_{Al}\approx9,541\%\\ b,n_{Cu}=\dfrac{12,8}{64}=0,2\left(mol\right)\\ n_{H_2}=\dfrac{3}{2}.n_{Al}=\dfrac{3}{2}.0,05=0,075\left(mol\right)\\ \Rightarrow V=V_{H_2\left(đktc\right)}=0,075.22,4=1,68\left(l\right)\\ 4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ 2Cu+O_2\rightarrow\left(t^o\right)2CuO\\ n_{O_2}=\dfrac{3}{4}.n_{Al}+\dfrac{1}{2}.n_{Cu}=\dfrac{3}{4}.0,05+\dfrac{1}{2}.0,2=0,0875\left(mol\right)\)
\(\Rightarrow V_{O_2\left(đktc\right)}=0,0875.22,4=1,96\left(l\right)\)

\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
Gọi \(\left\{{}\begin{matrix}m_{Mg}=a\\n_{Fe}=b\end{matrix}\right.\) => 24a + 56b = 20
PTHH: Mg + 2HCl --> MgCl2 + H2
______a------------------>a------>a__________(mol)
Fe + 2HCl --> FeCl2 + H2
_b----------------->b----->b__________________(mol)
=> a+b = 0,5
=> \(\left\{{}\begin{matrix}a=0,25\\b=0,25\end{matrix}\right.\) => \(\left\{{}\begin{matrix}m_{MgCl_2}=0,25.95=23,75\left(g\right)\\m_{FeCl_2}=0,25.127=31,75\left(g\right)\end{matrix}\right.\) => m muối = 55,5(g)

\(19,1gam\) \(:\left\{{}\begin{matrix}Al\\Mg\\Zn\end{matrix}\right.\)\(\underrightarrow{+O_2}\)\(Y:25,5gam\)\(\underrightarrow{+HCl}\left\{{}\begin{matrix}AgCl_3\\MgCl_2\\ZnCl_2\end{matrix}\right.\) + H2 : 0,3 mol
H2O
Áp dụng định luật bảo toàn khối lượng:
\(mO_2=25,5-19,1=6,4gam\) \(\Rightarrow nO_2=0,2\left(mol\right)\)
BTNT O : nH2O = 0,4mol
\(\rightarrow nHCl^-\left(tdOxi\right)=0,8\left(mol\right)\)
\(nH_2=0,3\left(mol\right)\rightarrow nCl^-\left(tdKl\right)=0,6\left(mol\right)\)
\(m_{muối}=19,1+\left(0,8+0,6\right).35,5=68,8\left(g\right)\)

Gọi \(\left\{{}\begin{matrix}n_{Mg}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow24a+56b=20\) (1)
Bảo toàn nguyên tố: \(n_{HCl}=2n_{MgCl_2}+2n_{FeCl_2}=2n_{Mg}+2n_{Fe}=2a+2b\)
\(\Rightarrow2a+2b=0,5\cdot2\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,25\\b=0,25\end{matrix}\right.\)
+) Trong không khí
Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,125\left(mol\right)\\n_{MgO}=n_{Mg}=0,25\left(mol\right)\end{matrix}\right.\) \(\Rightarrow m_1=0,125\cdot160+0,25\cdot40=30\left(g\right)\)
+) Trong chân không
Bảo toàn nguyên tố: \(n_{FeO}=n_{Fe}=n_{Mg}=n_{MgO}=0,25\left(mol\right)\)
\(\Rightarrow m_2=0,25\cdot72+0,25\cdot40=28\left(g\right)\)

Dung dịch X chứa :HCl ,FeCl2 ,CuCl2
\(n_{NO} = 0,05(mol)\)
Suy ra: \(n_{HCl\ dư} = 4n_{NO} = 0,2(mol)\)
\(\Rightarrow n_{HCl\ pư} = 0,8.1,25 - 0,2 = 0,8(mol)\\ 2H^+ + O^{2-} \to H_2O\)
Suy ra : \(n_{O(oxit)} = 0,5n_{H^+} = 0,4(mol)\)
BTNT với O : \(n_{Fe_3O_4} = \dfrac{1}{4} n_{Fe_3O_4} = 0,1(mol)\\ \Rightarrow n_{FeCl_2} = 3n_{Fe_3O_4} = 0,3(mol)\\ \Rightarrow n_{Ag} = 0,3(mol)\)
BTNT với Cl : \(n_{AgCl} = n_{HCl} = 1(mol)\)
Vậy m = 0,3.108 + 1.143,5 = 175,9(gam)