Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Cu}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
pthh: \(H_2+CuO\underrightarrow{t^o}Cu+H_2O\)
0,1 0,1 0,1
=>\(\left\{{}\begin{matrix}V_{H_2}=0,1.22,4=2,24\left(l\right)\\m_{H_2O}=0,1.18=1,8\left(g\right)\end{matrix}\right.\)

a.b.
\(n_{Fe}=\dfrac{2,8}{56}=0,05mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,05 0,1 0,05 ( mol )
\(V_{H_2}=0,05.22,4=1,12l\)
\(m_{HCl}=0,1.36,5=3,65g\)
c.
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,05 0,05 ( mol )
\(m_{Cu}=0,05.64=3,2g\)

Theo ĐLBTKL: mCuO + mH2 = mrắn sau pư + mH2O
Mà \(\left\{{}\begin{matrix}m_{H_2}=2a\left(g\right)\\m_{H_2O}=18a\left(g\right)\end{matrix}\right.\)
=> 32 + 2a = 28,8 + 18a ý bn :)
?????
MH2 = 2 (g/mol), nH2 = a (mol) thì mH2 = 2a (g) còn gì
tương tự với H2O

\(n_{Fe_2O_3}=\dfrac{32}{160}=0.2\left(mol\right)\)
\(Fe_2O_3+3H_2\underrightarrow{^{^{t^o}}}2Fe+3H_2O\)
\(0.2........0.6........0.4........0.6\)
\(V_{H_2}=0.6\cdot22.4=13.44\left(l\right)\)
\(m_{Fe}=0.4\cdot56=22.4\left(g\right)\)
Số phân tử H2O là : \(0.6\cdot6\cdot10^{23}=3.6\cdot10^{23}\left(pt\right)\)

a)PTHH: CuO+H2=>Cu+H2O
b) nCu=0,05g
PTHH: CuO+H2=>Cu+H2O
0,05<-0,05<-0,05->0,05
=> VH2 =0,05.22,4=1,12 lit

a)\(n_{H_2}=\dfrac{8,96}{22,4}=0,4mol\)
\(2H_2+O_2\underrightarrow{t^o}2H_2O\)
0,4 0,2 0,4
\(V_{O_2}=0,2\cdot22,4=4,48l\)
\(V_{kk}=5V_{O_2}=5\cdot4,48=22,4l\)
b)\(CuO+H_2\rightarrow Cu+H_2O\)
0,4 0,4
\(m_{H_2O}=0,4\cdot18=7,2g\)

\(n_{Cu}=\dfrac{6,4}{64}=0,1mol\)
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,1 0,1 ( mol )
\(\left\{{}\begin{matrix}m_{CuO}=0,1.80=8g\\m_{FeO}=12-8=4g\end{matrix}\right.\)

a)
FeO + H2 --to--> Fe + H2O
CuO + H2 --to--> Cu + H2O
b) \(n_{Cu}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
0,1<--0,1<-----0,1
=> \(m_{FeO}=12-0,1.80=4\left(g\right)\)
=> \(n_{FeO}=\dfrac{4}{72}=\dfrac{1}{18}\left(mol\right)\)
FeO + H2 --to--> Fe + H2O
\(\dfrac{1}{18}\)-->\(\dfrac{1}{18}\)----->\(\dfrac{1}{18}\)
=> \(V_{H_2}=\left(0,1+\dfrac{1}{18}\right).22,4=\dfrac{784}{225}\left(l\right)\)
c) \(m_{Fe}=\dfrac{1}{18}.56=\dfrac{28}{9}\left(g\right)\)
d) \(\left\{{}\begin{matrix}m_{CuO}=0,1.80=8\left(g\right)\\m_{FeO}=4\left(g\right)\end{matrix}\right.\)
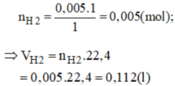
a)PTHH: CuO+H2=>Cu+H2O
nCu=0,05g
PTHH: CuO+H2=>Cu+H2O
0,05<-0,05<-0,05->0,05
=> VH2 =0,05.22,4=1,12 lit
nO2=2,2422,4=0,1(mol)nO2=2,2422,4=0,1(mol)
PTHH: 2Cu + O2 --to--> 2CuO
0,1-------->0,2
=> mCuO = 0,2.80 = 16(g)