Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Ta có: \(n_K=\dfrac{7,8}{39}=0,2\left(mol\right)\)
a, PT: \(2K+2CH_3COOH\rightarrow2CH_3COOK+H_2\)
_____0,2______0,2_____________________0,1 (mol)
b, \(m_{CH_3COOH}=0,2.60=12\left(g\right)\)
c, \(V_{H_2}=0,1.22,4=2,24\left(l\right)\)
Bạn tham khảo nhé!

n$Mg$ =4,8/24=0,2 mol
n$CH3COOH$ =12/60=0,2 mol
Xét tỉ lệ mol=>$CH3COOH$ hết
2 $CH3COOH$ +$Mg$ => $(CH3COO)2Mg$ + $H_2$
0,2 mol =>0,1 mol
V$H_2$ =0,1.22,4=2,24l

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
\(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
0,1 0,2
a. \(V_{CH_3COOH}=\dfrac{0,2}{1}=0,2\left(l\right)\)
b. \(CH_3COOH+C_2H_5OH⇌\left(H_2SO_{4đ},t^o\right)CH_3COOC_2H_5+H_2O\)
0,2 0,2
Với H% = 80
\(m_{CH_3COOC_2H_5}=\dfrac{0,2.88.80}{100}=14,08\left(g\right)\)

a, \(Na_2CO_3+2HCl\rightarrow2NaCl+CO_2+H_2O\)
b,\(n_{Na_2CO_3}=\dfrac{21,2}{106}=0,2\left(mol\right)\)
Theo PT: \(n_{HCl}=2n_{Na_2CO_3}=0,4\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
c, \(n_{CO_2}=n_{Na_2CO_3}=0,2\left(mol\right)\)
\(\Rightarrow V_{CO_2}=0,2.22,4=4,48\left(l\right)\)

\(n_{Na}=\dfrac{9,2}{23}=0,4mol\)
\(Na+CH_3COOH\rightarrow CH_3COONa+\dfrac{1}{2}H_2\)
0,4 0,4 ( mol )
\(m_{CH_3COOH}=0,4.60=24g\)

nCH3COOH=48/60=0,8 mol
2CH3COOH + Fe --> (CH3COO)2Fe + H2
0,8 0,4 0,4 mol
=>m(CH3COO)2Fe=0,4*174=69,6 g
2H2 +O2 --> 2H2O
0,4 0,2 mol
=>VO2=0,2*22,4=4,48 lít
=> V không khí =4,48*5=22,4 lít
PT: \(2CH_3COOH+Fe\rightarrow\left(CH_3COO\right)_2Fe+H_2\)
a, Ta có: \(n_{CH_3COOH}=\dfrac{4,8}{60}=0,08\left(mol\right)\)
Theo PT: \(n_{\left(CH_3COO\right)_2Fe}=\dfrac{1}{2}n_{CH_3COOH}=0,04\left(mol\right)\)
\(\Rightarrow m_{\left(CH_3COO\right)_2Fe}=0,04.174=6,96\left(g\right)\)
b, Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{CH_3COOH}=0,04\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
___0,04__0,02 (mol)
\(\Rightarrow V_{O_2}=0,02.22,4=0,448\left(l\right)\)
\(\Rightarrow V_{kk}=0,448.5=2,24\left(l\right)\)
Bạn tham khảo nhé!
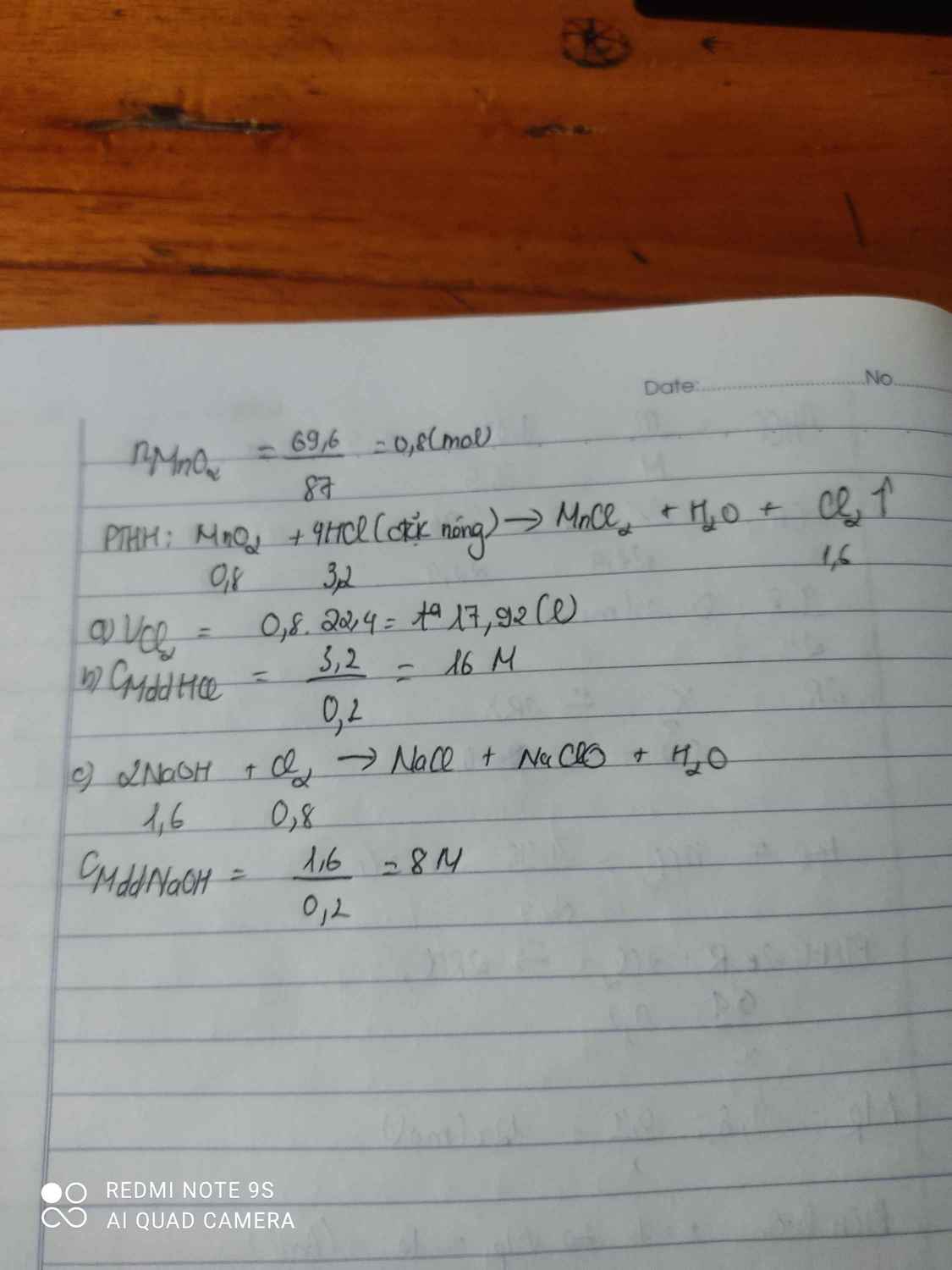
$a\big)$
$n_{Na}=\dfrac{4,6}{23}=0,2(mol)$
$CH_3COOH+Na\to CH_3COONa+\dfrac{1}{2}H_2$
Theo PT: $n_{CH_3COOH}=n_{Na}=0,2(mol)$
$\to m_{CH_3COOH}=0,2.60=12(g)$
$b\big)$
Theo PT: $n_{H_2}=\dfrac{1}{2}n_{Na}=0,1(mol)$
$\to V_{H_2(đktc)}=0,1.22,4=2,24(l)$
giúp tuiii