Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,PTHH:2Na+2H_2O\rightarrow2NaOH+H_2\\ 2K+2H_2O\rightarrow2KOH+H_2\\ n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right);n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\\ b,n_{H_2\left(tổng\right)}=\dfrac{1}{2}.\left(n_{Na}+n_K\right)=\dfrac{0,2+0,1}{2}=0,15\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,2.22,4=4,48\left(l\right)\)

a) \(2Na+2H_2O\rightarrow2NaOH+H_2\left(1\right)\)
\(2K+2H_2O\rightarrow2KOH+H_2\left(2\right)\)
b) \(n_{Na}=\frac{4,6}{23}=0,2\left(mol\right)\)
Theo PTHH (1): \(n_{Na}:n_{H_2}=2:1\)
\(\Rightarrow n_{H_2\left(1\right)}=n_{Na}.\frac{1}{2}=0,2.\frac{1}{2}=0,1\left(mol\right)\)
\(\Rightarrow V_{H_2\left(1\right)}=0,1.22,4=2,24\left(l\right)\)
\(n_K=\frac{3,9}{39}=0,1\left(mol\right)\)
Theo PTHH (2): \(n_K:n_{H_2}=2:1\)
\(\Rightarrow n_{H_2\left(2\right)}=n_K.\frac{1}{2}=0,1.\frac{1}{2}=0,05\left(mol\right)\)
\(\Rightarrow V_{H_2\left(2\right)}=0,05.22,4=1,12\left(l\right)\)
\(\Rightarrow V_{h^2}=2,24+1,12=3,36\left(l\right)\)
c) Dung dịch thu được sau phản ứng làm giấy quỳ tím chuyển đổi thành màu xanh vì nó là dung dịch bazơ.

1)
a) Kim loại tan dần, chạy tròn trên mặt nước, xuất hiện khí không màu không mùi.
$2Na + 2H_2O \to 2NaOH + H_2$
$2K + 2H_2O \to 2KOH + H_2$
b) n Na = 4,6/23 = 0,2(mol) ; n K = 3,9/39 = 0,1(mol)
n H2 = 1/2 n Na + 1/2 n K = 0,15(mol)
V H2 = 0,15.22,4 = 3,36 lít
2)
a)
$Ba + 2H_2O \to Ba(OH)_2 + H_2$
$H_2 + CuO \xrightarrow{t^o} Cu + H_2O$
b) n H2 = n Ba = 6,85/137 = 0,05(mol)
V H2 = 0,05.22,4 = 1,12(lít)
c)
Ta thấy :
n CuO / 1 = 8/80 = 0,1(mol) < n H2 / 1 = 0,05 nên CuO dư
n CuO pư = n Cu = n H2 = 0,05(mol)
Suy ra :
m = m CuO dư + m Cu = (8 - 0,05.80) + 0,05.64 = 7,2(gam)

`2Na+2H_2O->2NaOH+H_2`
x-----------------------------`1/2`x mol
`2K+2H_2O->2KOH+H_2`
y---------------------------`1/2` y mol
`n_(H_2)=(6,72)/(22,4)=0,3 mol`
Ta có phương trình :
\(\left\{{}\begin{matrix}23x+39y=9,3\\\dfrac{1}{2}x+\dfrac{1}{2}y=0,3\end{matrix}\right.\)
-> nghiệm vô lí
`#YBTran~`

\(a) Mg + 2HCl \to MgCl_2 + H_2\\ b) n_{MgCl_2} = n_{Mg} = \dfrac{0,24}{24} = 0,01(mol)\\ m_{MgCl_2} = 0,01.95 = 0,95(gam)\\ c) n_{H_2} = n_{Mg} = 0,01(mol) \Rightarrow V_{H_2} = 0,01.22,4 = 0,224(lít)\)

a,\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right);n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
PTHH: 2Na + 2H2O → 2NaOH + H2
Mol: 0,2 0,1
PTHH: 2K + 2H2O → 2KOH + H2
Mol: 0,1 0,05
b, \(n_{H_2}=0,1+0,05=0,15\left(mol\right)\)
\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
c,mdd sau pứ=4,6+3,9+91,5-0,15.2=99,7 (g)
\(\%m_{NaOH}=\dfrac{0,2.40.100\%}{99,7}=8,02\%\)
\(\%m_{KOH}=\dfrac{0,1.56.100\%}{99,7}=5,62\%\)
Bài 3 :
\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
\(n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
a) Pt : \(2Na+2H_2O\rightarrow2NaOH+H_2|\)
2 2 2 1
0,2 0,2 0,1
\(2K+2H_2O\rightarrow2KOH+H_2|\)
2 2 2 1
0,1 0,1 0,05
b) \(n_{H2\left(tổng\right)}=0,1+0,05=0,15\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\)
c) \(n_{NaOH}=\dfrac{0,1.2}{1}=0,2\left(mol\right)\)
⇒ \(m_{NaOH}=0,2.40=8\left(g\right)\)
\(n_{KOH}=\dfrac{0,05.2}{1}=0,1\left(mol\right)\)
⇒ \(m_{KOH}=0,1.56=5,6\left(g\right)\)
\(m_{ddspu}=8,5+91,5-\left(0,15.2\right)=99,7\left(g\right)\)
\(C_{NaOH}=\dfrac{8.100}{99,7}=8,02\)0/0
\(C_{KOH}=\dfrac{5,6.100}{99,7}=5,62\)0/0
Chúc bạn học tốt

\(n_{Na}=\dfrac{4.6}{23}=0.2\left(mol\right)\)
\(n_K=\dfrac{3.9}{39}=0.1\left(mol\right)\)
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
\(K+H_2O\rightarrow KOH+\dfrac{1}{2}H_2\)
\(V_{H_2}=\left(\dfrac{0.2}{2}+\dfrac{0.1}{2}\right)\cdot22.4=3.36\left(l\right)\)
\(m_{bazo}=0.2\cdot40+0.1\cdot56=13.6\left(g\right)\)

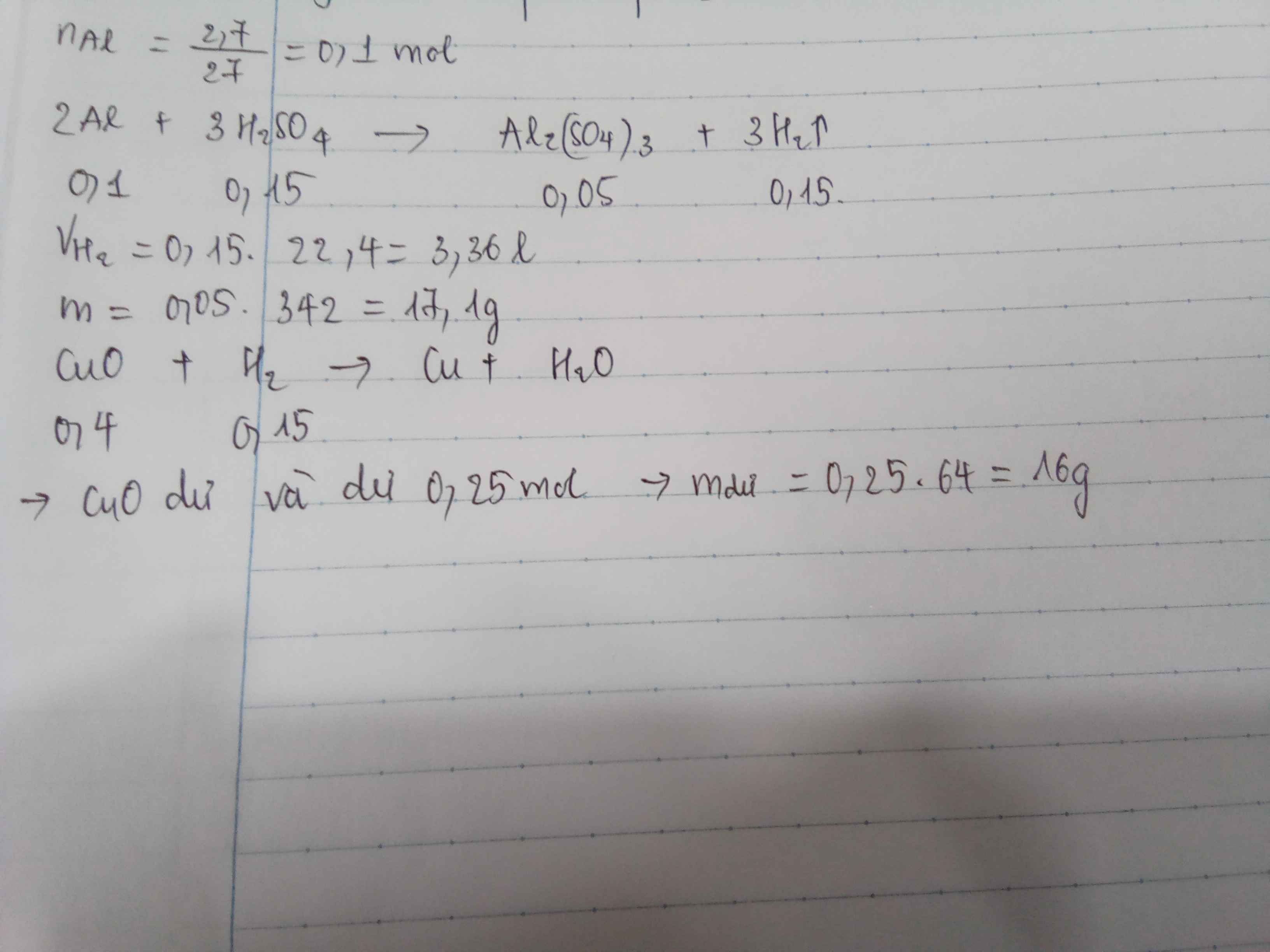
\(n_{Na}=\dfrac{4.6}{23}=0.2\left(mol\right)\)
\(n_K=\dfrac{3.9}{39}=0.1\left(mol\right)\)
\(Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
\(K+H_2O\rightarrow KOH+\dfrac{1}{2}H_2\)
\(n_{H_2}=0.1+0.05=0.15\left(mol\right)\)
\(V_{H_2}=0.15\cdot22.4=3.36\left(l\right)\)
a) nNa=4,6/23=0,2(mol)
nK=3,9/39=0,1(mol)
PTHH: 2 Na + 2 H2O -> 2 NaOH + H2
0,2____________0,2______0,2__0,1(mol)
2 K + 2 H2O -> 2 KOH + H2
0,1____0,1______0,1___0,05(mol)
b) V(H2,đktc)=(0,05+0,1).22,4=3,36(l)